Effectiveness of aromatase inhibitors and tamoxifen in reducing subsequent breast cancer
- PMID: 23342281
- PMCID: PMC3544463
- DOI: 10.1002/cam4.37
Effectiveness of aromatase inhibitors and tamoxifen in reducing subsequent breast cancer
Abstract
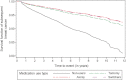
Tamoxifen (TAM) has been prescribed for decades and aromatase inhibitors (AIs) have been used since the early 2000s in preventing subsequent breast cancer. However, outside of clinical trials, the effectiveness of AIs is not established. We examined the long-term risk of subsequent breast cancer among survivors treated with TAM and AIs in a large health plan. The study included 22,850 survivors, diagnosed with initial breast cancer (stages 0-IV) from 1996 to 2006, and followed 13 years maximum. We compared the risk of subsequent breast cancer in those who used TAM and/or AIs versus nonusers (the reference group). Hazard ratios (HR) adjusted for patient, tumor, treatment, and health-care characteristics were estimated using Cox models with time-dependent drug use status. Women who used TAM/AIs had a large reduction in risk of subsequent breast cancer compared with nonusers. While confidence intervals (CI) for all hormone treatment groups overlapped, women with high adherence (medication possession ratio ≥80%) who used AIs exclusively and had positive ER or PR receptor status had the greatest risk reduction (HR = 0.34, 95% CI: 0.28-0.41), followed by those who switched from TAM to AIs (HR = 0.39, 95% CI: 0.30-0.49), and those who used TAM exclusively (HR = 0.42, 95% CI: 0.36-0.47). Women with high adherence had the greatest risk reduction in subsequent breast cancer, but the results were not substantially different from women who took the drugs less regularly. Compared with nonusers, the reduction in subsequent breast cancer risk ranged from 58% to 66% across the hormone treatment groups and degree of adherence.
Keywords: AI; breast cancer; comparative effectiveness; tamoxifen.
Figures
References
-
- American Cancer Society. Cancer facts and figures 2012. Am. Cancer Soc. 2012 Available at http://www.cancer.org. Accessed 3 February 2012.
-
- Hewitt M, Greenfield S, Stoval E. From cancer patient to cancer survivor: lost in translation. Washington, DC: The National Academy Press; 2006.
-
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thürlimann B, Senn HJ, Panel members Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann. Oncol. 2005;16:1569–1583. - PubMed
-
- Arora A, Potter JF. Aromatase inhibitors: current indications and future prospects for treatment of postmenopausal breast cancer. J. Am. Geriatr. Soc. 2004;52:611–616. - PubMed
-
- Winer EP, Hudis C, Burstein HJ, Bryant J, Chlebowski RT, Ingle JN, et al. American Society of Clinical Oncology technology assessment working group update: use of aromatase inhibitors in the adjuvant setting. J. Clin. Oncol. 2003;21:2597–2599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials