Group 10 allergens (tropomyosins) from house-dust mites may cause covariation of sensitization to allergens from other invertebrates
- PMID: 23342293
- PMCID: PMC3548612
- DOI: 10.2500/ar.2012.3.0036
Group 10 allergens (tropomyosins) from house-dust mites may cause covariation of sensitization to allergens from other invertebrates
Abstract
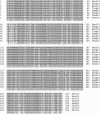
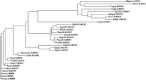
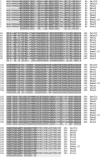
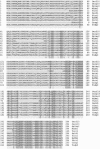
Group 10 allergens (tropomyosins) have been assumed to be a major cause of cross-reactivity between house-dust mites (HDMs) and other invertebrates. Despite all of the published data regarding the epidemiology, percent IgE binding and level of sensitization in the population, the role of tropomyosin as a cross-reactive allergen in patients with multiple allergy syndrome still remains to be elucidated. Homology between amino acid sequences reported in allergen databases of selected invertebrate tropomyosins was determined with Der f 10 as the reference allergen. The 66.9 and 54.4% identities were found with selected crustacean and insect species, respectively, whereas only 20.4% identity was seen with mollusks. A similar analysis was performed using reported B-cell IgE-binding epitopes from Met e1 (shrimp allergen) and Bla g7 (cockroach allergen) with other invertebrate tropomyosins. The percent identity in linear sequences was higher than 35% in mites, crustaceans, and cockroaches. The polar and hydrophobic regions in these groups were highly conserved. These findings suggest that tropomyosin may be a major cause of covariation of sensitization between HDMs, crustaceans, and some species of insects and mollusks.
Keywords: Cross-reactivity of tropomyosins; HDM allergens; IgE-binding epitopes; group 10 allergens; homology; multiple allergy syndrome; tropomyosins.
Conflict of interest statement
The authors have no conflicts of interest to declare pertaining to this article
Figures



Similar articles
-
Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins.Int Arch Allergy Immunol. 2002 Sep;129(1):38-48. doi: 10.1159/000065172. Int Arch Allergy Immunol. 2002. PMID: 12372997
-
Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin).Int Arch Allergy Immunol. 2002 Jan;127(1):27-37. doi: 10.1159/000048166. Int Arch Allergy Immunol. 2002. PMID: 11893851
-
Shellfish and House Dust Mite Allergies: Is the Link Tropomyosin?Allergy Asthma Immunol Res. 2016 Mar;8(2):101-6. doi: 10.4168/aair.2016.8.2.101. Epub 2015 Jul 14. Allergy Asthma Immunol Res. 2016. PMID: 26739402 Free PMC article. Review.
-
Tropomyosin: an invertebrate pan-allergen.Int Arch Allergy Immunol. 1999 Aug;119(4):247-58. doi: 10.1159/000024201. Int Arch Allergy Immunol. 1999. PMID: 10474029 Review.
-
Cockroach allergens and asthma in Brazil: identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens.J Allergy Clin Immunol. 1999 Aug;104(2 Pt 1):329-37. doi: 10.1016/s0091-6749(99)70375-1. J Allergy Clin Immunol. 1999. PMID: 10452753
Cited by
-
Bypassing the build-up phase for oral immunotherapy in shrimp-allergic children.World Allergy Organ J. 2024 Feb 3;17(2):100865. doi: 10.1016/j.waojou.2023.100865. eCollection 2024 Feb. World Allergy Organ J. 2024. PMID: 38351903 Free PMC article.
-
Insights into the Allergenic Potential of the Edible Yellow Mealworm (Tenebrio molitor).Foods. 2019 Oct 18;8(10):515. doi: 10.3390/foods8100515. Foods. 2019. PMID: 31635354 Free PMC article.
-
Can Physicochemical Properties Alter the Potency of Aeroallergens? Part 1 - Aeroallergen Protein Families.Curr Allergy Asthma Rep. 2024 Nov;24(11):591-607. doi: 10.1007/s11882-024-01172-8. Epub 2024 Sep 20. Curr Allergy Asthma Rep. 2024. PMID: 39302571 Free PMC article. Review.
-
Ion-Exchange Chromatography Coupled With Dynamic Coating Capillary Electrophoresis for Simultaneous Determination of Tropomyosin and Arginine Kinase in Shellfish.Front Chem. 2018 Jul 25;6:305. doi: 10.3389/fchem.2018.00305. eCollection 2018. Front Chem. 2018. PMID: 30090807 Free PMC article.
-
Cross-reactivity between aeroallergens and food allergens.World J Methodol. 2015 Jun 26;5(2):31-50. doi: 10.5662/wjm.v5.i2.31. eCollection 2015 Jun 26. World J Methodol. 2015. PMID: 26140270 Free PMC article.
References
-
- Platts-Mills TA, Vervloet D, Thomas WR, et al. Indoor allergens and asthma: Report of the Third International Workshop. J Allergy Clin Immunol 100:2–24, 1997 - PubMed
-
- Arlian LG, Geis DP, Vyszenski-Moher DL, et al. Antigenic and allergenic properties of the storage mite Tyrophagus putrescentiae. J Allergy Clin Immunol 74:166–172, 1984 - PubMed
-
- Arlian LG, Morgan MS, Neal JS. Dust mite allergens: Ecology and distribution. Curr Allergy Asthma Rep 2:401–411, 2002 - PubMed
-
- Leung R, Jenkins M. Asthma, allergy and atopy in southern Chinese school students. Clin Exp Allergy 24:353–358, 1994 - PubMed
-
- Arlian LG, Platts-Mills TAE. The biology of dust mites and the remediation of mite allergens in allergic disease. J Allergy Clin Immunol 107(suppl):S406–S413, 2001 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

