Utility of 3'-[(18)F]fluoro-3'-deoxythymidine as a PET tracer to monitor response to gene therapy in a xenograft model of head and neck carcinoma
- PMID: 23342298
- PMCID: PMC3545366
Utility of 3'-[(18)F]fluoro-3'-deoxythymidine as a PET tracer to monitor response to gene therapy in a xenograft model of head and neck carcinoma
Abstract
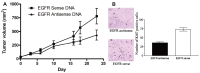
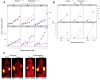
Noninvasive imaging methodologies are needed to assess treatment responses to novel molecular targeting approaches for the treatment of squamous cell carcinoma of the head and neck (SCCHN). Computer tomography and magnetic resonance imaging do not effectively distinguish tumors from fibrotic tissue commonly associated with SCCHN tumors. Positron emission tomography (PET) offers functional non-invasive imaging of tumors. We determined the uptake of the PET tracers 2-deoxy-2-[(18)F]fluoro-D-glucose ([(18)F]FDG) and 3'-[(18)F]Fluoro-3'-deoxythymidine ([(18)F]FLT) in several SCCHN xenograft models. In addition, we evaluated the utility of [(18)F]FLT microPET imaging in monitoring treatment response to an EGFR antisense approach targeted therapy that has shown safety and efficacy in a phase I trial. Two of the 3 SCCHN xenograft models tested demonstrated no appreciable uptake or retention of [(18)F]FDG, but consistent accumulation of [(18)F]FLT. The third tumor xenograft SCCHN model (Cal33) demonstrated variable uptake of both tracers. SCCHN xenografts (1483) treated with EGFR antisense gene therapy decreased tumor volumes in 4/6 mice. Reduced uptake of [(18)F]FLT was observed in tumors that responded to epidermal growth factor antisense (EGFRAS) gene therapy compared to non-responding tumors or tumors treated with control sense plasmid DNA. These findings indicate that [(18)F]FLT PET imaging may be useful in monitoring SCCHN response to molecular targeted therapies, while [(18)F]FDG uptake in SCCHN xenografts may not be reflective of the level of metabolic activity characteristic of human SCCHN tumors.
Keywords: 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG); 3’-[18F]Fluoro-3’-deoxythymidine ([18F]FLT); Squamous cell carcinoma of the head and neck (SCCHN); epidermal growth factor receptor (EGFR); positron emission tomography (PET); region of interest (ROI); standardize uptake values (SUV); volume of interest (VOI).
Figures


Similar articles
-
Monitoring response to radiotherapy in human squamous cell cancer bearing nude mice: comparison of 2'-deoxy-2'-[18F]fluoro-D-glucose (FDG) and 3'-[18F]fluoro-3'-deoxythymidine (FLT).Mol Imaging Biol. 2007 Nov-Dec;9(6):340-7. doi: 10.1007/s11307-007-0104-5. Mol Imaging Biol. 2007. PMID: 17643202 Free PMC article.
-
Can 3'-Deoxy-3'-((18)F) Fluorothymidine Out Perform 2-Deoxy-2-((18)F) Fluoro-D-Glucose Positron Emission Tomography/Computed Tomography in the Diagnosis of Cervical Lymphadenopathy in Patients With Oral/Head and Neck Cancer?J Oral Maxillofac Surg. 2015 Jul;73(7):1420-8. doi: 10.1016/j.joms.2015.01.002. Epub 2015 Jan 13. J Oral Maxillofac Surg. 2015. PMID: 25869746
-
Early detection of erlotinib treatment response in NSCLC by 3'-deoxy-3'-[F]-fluoro-L-thymidine ([F]FLT) positron emission tomography (PET).PLoS One. 2008;3(12):e3908. doi: 10.1371/journal.pone.0003908. Epub 2008 Dec 12. PLoS One. 2008. PMID: 19079597 Free PMC article.
-
Monitoring of anti-cancer treatment with (18)F-FDG and (18)F-FLT PET: a comprehensive review of pre-clinical studies.Am J Nucl Med Mol Imaging. 2015 Oct 12;5(5):431-56. eCollection 2015. Am J Nucl Med Mol Imaging. 2015. PMID: 26550536 Free PMC article. Review.
-
Current clinical status of 18F-FLT PET or PET/CT in digestive and abdominal organ oncology.Abdom Radiol (NY). 2017 Mar;42(3):951-961. doi: 10.1007/s00261-016-0947-9. Abdom Radiol (NY). 2017. PMID: 27770160 Review.
Cited by
-
Multimodality imaging of RNA interference.Curr Med Chem. 2013;20(29):3664-75. doi: 10.2174/0929867311320290012. Curr Med Chem. 2013. PMID: 23745567 Free PMC article. Review.
-
Utility of [18 F]FLT-PET to assess treatment response in trastuzumab-resistant and trastuzumab-sensitive HER2-overexpressing human breast cancer xenografts.Mol Imaging Biol. 2015 Feb;17(1):119-28. doi: 10.1007/s11307-014-0770-z. Mol Imaging Biol. 2015. PMID: 25034624 Free PMC article.
-
18F-FLT and 18F-FDG PET-CT imaging in the evaluation of early therapeutic effects of chemotherapy on Walker 256 tumor-bearing rats.Exp Ther Med. 2016 Dec;12(6):4154-4158. doi: 10.3892/etm.2016.3869. Epub 2016 Nov 3. Exp Ther Med. 2016. PMID: 28101193 Free PMC article.
-
In vitro measurement of glucose uptake after radiation and cetuximab treatment in head and neck cancer cell lines using 18F-FDG, gamma spectrometry and PET/CT.Oncol Lett. 2019 Nov;18(5):5155-5162. doi: 10.3892/ol.2019.10916. Epub 2019 Sep 24. Oncol Lett. 2019. PMID: 31620196 Free PMC article.
-
In vivo evaluation of the effects of simultaneous inhibition of GLUT-1 and HIF-1α by antisense oligodeoxynucleotides on the radiosensitivity of laryngeal carcinoma using micro 18F-FDG PET/CT.Oncotarget. 2017 May 23;8(21):34709-34726. doi: 10.18632/oncotarget.16671. Oncotarget. 2017. PMID: 28410229 Free PMC article.
References
-
- Anzai Y, Carroll WR, Quint DJ, Bradford CR, Minoshima S, Wolf GT, Wahl RL. Recurrence of head and neck cancer after surgery or irradiation: prospective comparison of 2-deoxy-2-[F-18] fluoro-D-glucose PET and MR imaging diagnoses. Radiology. 1996;200:135–141. - PubMed
-
- Greven KM, Williams DW 3rd, Keyes JW Jr, Mc-Guirt WF, Watson NE Jr, Randall ME, Raben M, Geisinger KR, Cappellari JO. Positron emission tomography of patients with head and neck carcinoma before and after high dose irradiation. Cancer. 1994;74:1355–1359. - PubMed
-
- Dobert N, Kovacs AF, Menzel C, Hamscho N, Yuen Yuen H, Engels K, Walendzik H, Grunwald F. The prognostic value of FDG PET in head and neck cancer. Correlation with histopathology. Q J Nucl Med Mol Imaging. 2005;49:253–257. - PubMed
-
- Nam SY, Lee SW, Im KC, Kim JS, Kim SY, Choi SH, Ryu JS, Moon DH, Oh SJ, Yi BY, Kim JH, Ahn SD, Shin SS, Kim SB, Choi EK, Lee BJ. Early evaluation of the response to radiotherapy of patients with squamous cell carcinoma of the head and neck using 18FDG-PET. Oral Oncol. 2005;41:390–395. - PubMed
-
- Kim SY, Lee SW, Nam SY, Im KC, Kim JS, Oh SJ, Ahn SD, Shin SS, Choi EK, Kim JH. The Feasibility of 18F-FDG PET scans 1 month after completing radiotherapy of squamous cell carcinoma of the head and neck. J Nucl Med. 2007;48:373–378. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
