Nanomedicine for uterine leiomyoma therapy
- PMID: 23343157
- PMCID: PMC3754434
- DOI: 10.4155/tde.12.144
Nanomedicine for uterine leiomyoma therapy
Abstract
Background: The purpose of this work was to engineer polymeric nanoparticles to encapsulate and deliver 2-methoxyestradiol, a potential antitumor drug for treatment of uterine leiomyoma (fibroids), the most common hormone-dependent pathology affecting women of reproductive age.
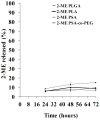
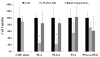
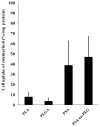
Methods/results: Encapsulation efficiency and drug release from the nanoparticles were monitored by HPLC. Cell morphology and in vitro cytotoxicity experiments were carried out in a human leiomyoma cell line. The nanoparticles displayed high encapsulation efficiency (>86%), which was verified by differential scanning calorimetry and x-ray diffraction. Excellent long-term stability of the nanoparticles and gradual drug release without burst were also observed. Cellular uptake of fluorescent nanoparticles was confirmed by confocal imaging. The drug-loaded poly(lactic acid) and poly(lactic-co-glycolic acid) nanoparticles induced cytotoxicity in human leiomyoma cells to a significantly greater extent than the free drug at 0.35 µM.
Conclusion: This novel approach represents a potential fertility-preserving alternative to hysterectomy.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. Uterine leiomyoma results in heavy bleeding during menstruation for longer periods of times. Hystrectomy is considered one of the treatment options. - PubMed
-
- Al-Hendy A, Salama SA. Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. J Soc Gynecol Investig. 2006;13(2):136–144. - PubMed
-
- Martel KM, Ko AC, Christman GM, Stribley JM. Apoptosis in human uterine leiomyomas. Semin Reprod Med. 2004;22(2):91–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
