Plasma membrane events associated with the meiotic divisions in the amphibian oocyte: insights into the evolution of insulin transduction systems and cell signaling
- PMID: 23343451
- PMCID: PMC3577484
- DOI: 10.1186/1471-213X-13-3
Plasma membrane events associated with the meiotic divisions in the amphibian oocyte: insights into the evolution of insulin transduction systems and cell signaling
Abstract
Background: Insulin and its plasma membrane receptor constitute an ancient response system critical to cell growth and differentiation. Studies using intact Rana pipiens oocytes have shown that insulin can act at receptors on the oocyte surface to initiate resumption of the first meiotic division. We have reexamined the insulin-induced cascade of electrical and ion transport-related plasma membrane events using both oocytes and intact plasma membranes in order to characterize the insulin receptor-steroid response system associated with the meiotic divisions.
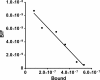
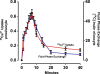
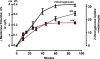
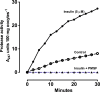
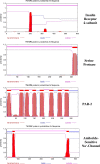
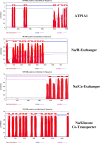
Results: [(125)I]Insulin binding (K(d) = 54 ± 6 nM) at the oocyte plasma membrane activates membrane serine protease(s), followed by the loss of low affinity ouabain binding sites, with a concomitant 3-4 fold increase in high affinity ouabain binding sites. The changes in protease activity and ouabain binding are associated with increased Na(+)/Ca2(+) exchange, increased endocytosis, decreased Na(+) conductance resulting in membrane hyperpolarization, increased 2-deoxy-D-glucose uptake and a sustained elevation of intracellular pH (pHi). Hyperpolarization is largely due to Na(+)-channel inactivation and is the main driving force for glucose uptake by the oocyte via Na(+)/glucose cotransport. The Na(+) sym- and antiporter systems are driven by the Na(+) free energy gradient generated by Na(+)/K(+)-ATPase. Shifts in α and/or β Na(+)-pump subunits to caveolar (lipid raft) membrane regions may activate Na/K-ATPase and contribute to the Na(+) free energy gradient and the increase in both Na(+)/glucose co-transport and pHi.
Conclusions: Under physiological conditions, resumption of meiosis results from the concerted action of insulin and progesterone at the cell membrane. Insulin inactivates Na(+) channels and mobilizes fully functional Na(+)-pumps, generating a Na(+) free energy gradient which serves as the energy source for several membrane anti- and symporter systems.
Figures

Similar articles
-
Studies of insulin action on the amphibian oocyte plasma membrane using NMR, electrophysiological and ion flux techniques.Biochim Biophys Acta. 1985 Mar 21;844(3):377-92. doi: 10.1016/0167-4889(85)90140-5. Biochim Biophys Acta. 1985. PMID: 3882159
-
The steroid-binding subunit of the Na/K-ATPase as a progesterone receptor on the amphibian oocyte plasma membrane.Steroids. 2005 Dec 15;70(14):933-45. doi: 10.1016/j.steroids.2005.07.002. Epub 2005 Sep 13. Steroids. 2005. PMID: 16165176
-
Caveolin-Na/K-ATPase interactions: role of transmembrane topology in non-genomic steroid signal transduction.Steroids. 2012 Sep;77(11):1160-8. doi: 10.1016/j.steroids.2012.04.012. Epub 2012 May 9. Steroids. 2012. PMID: 22579740
-
Progesterone induces meiotic division in the amphibian oocyte by releasing lipid second messengers from the plasma membrane.Steroids. 1999 Jan-Feb;64(1-2):157-67. doi: 10.1016/s0039-128x(98)00093-2. Steroids. 1999. PMID: 10323685 Review.
-
Role of G-protein-coupled estrogen receptor (GPER/GPR30) in maintenance of meiotic arrest in fish oocytes.J Steroid Biochem Mol Biol. 2017 Mar;167:153-161. doi: 10.1016/j.jsbmb.2016.12.005. Epub 2016 Dec 19. J Steroid Biochem Mol Biol. 2017. PMID: 28007532 Review.
Cited by
-
Identification of early transcriptome signatures in placenta exposed to insulin and obesity.Am J Obstet Gynecol. 2015 May;212(5):647.e1-11. doi: 10.1016/j.ajog.2015.02.026. Epub 2015 Feb 28. Am J Obstet Gynecol. 2015. PMID: 25731694 Free PMC article.
References
-
- Ebbererink RHM, Smit AB, Van Minnen J. The insulin family: evolution of structure and function in vertebrates and invertebrates. Biol Bull. 1989;177:176–182. doi: 10.2307/1541928. - DOI
-
- Rhodes CJ, White MF. Molecular insights into insulin action and secretion. Eur J Clin Invest. 2002;32:3–13. - PubMed
-
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nature Rev Mol Cell Biol. 2006;7:85–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

