Eastern equine encephalitis in children, Massachusetts and New Hampshire,USA, 1970-2010
- PMID: 23343480
- PMCID: PMC3559032
- DOI: 10.3201/eid1902.120039
Eastern equine encephalitis in children, Massachusetts and New Hampshire,USA, 1970-2010
Abstract
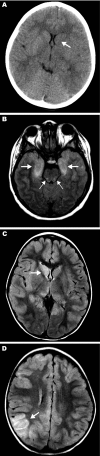
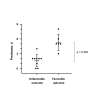
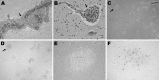
We describe the clinical, laboratory, and radiographic characteristics of 15 cases of eastern equine encephalitis in children during 1970-2010. The most common clinical and laboratory features were fever, headache, seizures, peripheral leukocytosis, and cerebrospinal fluid neutrophilic pleocytosis. Radiographic lesions were found in the basal ganglia, thalami, and cerebral cortex. Clinical outcomes included severe neurologic deficits in 5 (33%) patients, death of 4 (27%), full recovery of 4 (27%), and mild neurologic deficits in 2 (13%). We identify an association between a short prodrome and an increased risk for death or for severe disease.
Figures

References
-
- Centers for Disease Control and Prevention. Eastern equine encephalitis—New Hampshire and Massachusetts, August–September 2005. MMWR Morb Mortal Wkly Rep. 2006;55:697–700 . - PubMed
-
- Centers for Disease Control and Prevention. Eastern equine encephalitis: epidemiology and geographic distribution [cited 2012 Sept 25]. http://www.cdc.gov/EasternEquineEncephalitis/tech/epi.html#moremapschart...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
