ATM-dependent phosphorylation of heterogeneous nuclear ribonucleoprotein K promotes p53 transcriptional activation in response to DNA damage
- PMID: 23343766
- PMCID: PMC3594270
- DOI: 10.4161/cc.23592
ATM-dependent phosphorylation of heterogeneous nuclear ribonucleoprotein K promotes p53 transcriptional activation in response to DNA damage
Expression of concern in
-
Expression of Concern: ATM-dependent phosphorylation of heterogeneous nuclear ribonucleoprotein K promotes p53 transcriptional activation in response to DNA damage.Cell Cycle. 2024 Jul-Aug;23(13-16):892. doi: 10.1080/15384101.2025.2463772. Epub 2025 Mar 27. Cell Cycle. 2024. PMID: 40150821 Free PMC article. No abstract available.
Abstract
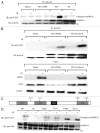
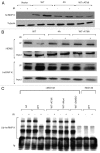
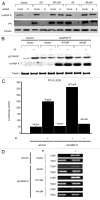
Previous work has established that heterogeneous nuclear ribonucleoprotein K (hnRNP K) is stabilized in an ATM-dependent manner in response to DNA damage and acts as a cofactor for p53-mediated transcription. Here, we show that in response to DNA damage caused by ionizing radiation, hnRNP K is phosphorylated in an ATM-dependent manner. Furthermore, our data indicate that ATM-dependent hnRNP K phosphorylation is required for its stabilization and its function as a p53 transcriptional cofactor in response to DNA damage. These findings thereby establish hnRNP K as an ATM target and help define how ATM orchestrates p53-dependent transcriptional responses in response to genotoxic stress.
Keywords: ATM; DNA damage; p53; phosphorylation; transcription.
Figures
Comment in
-
ATM targets hnRNPK to control p53.Cell Cycle. 2013 Apr 15;12(8):1162-3. doi: 10.4161/cc.24485. Epub 2013 Apr 2. Cell Cycle. 2013. PMID: 23549171 Free PMC article. No abstract available.
-
Fine-tuning the p53 response to DNA damage: a new piece in the puzzle.Cell Cycle. 2013 May 1;12(9):1337-8. doi: 10.4161/cc.24669. Epub 2013 Apr 15. Cell Cycle. 2013. PMID: 23588075 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
