Essential role of Cenexin1, but not Odf2, in ciliogenesis
- PMID: 23343771
- PMCID: PMC3594266
- DOI: 10.4161/cc.23585
Essential role of Cenexin1, but not Odf2, in ciliogenesis
Abstract
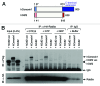
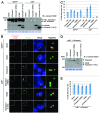
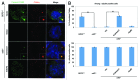
Primary cilia are microtubule-based solitary sensing structures on the cell surface that play crucial roles in cell signaling and development. Abnormal ciliary function leads to various human genetic disorders, collectively known as ciliopathies. Outer dense fiber protein 2 (Odf2) was initially isolated as a major component of sperm-tail fibers. Subsequent studies have demonstrated the existence of many splicing variants of Odf2, including Cenexin1 (Odf2 isoform 9), which bears an unusual C-terminal extension. Strikingly, Odf2 localizes along the axoneme of primary cilia, whereas Cenexin1 localizes to basal bodies in cultured mammalian cells. Whether Odf2 and Cenexin1 contribute to primary cilia assembly by carrying out either concerted or distinct functions is unknown. By taking advantage of odf2-/- cells lacking endogenous Odf2 and Cenexin1, but exogenously expressing one or both of these proteins, we showed that Cenexin1, but not Odf2, was necessary and sufficient to induce ciliogenesis. Furthermore, the Cenexin1-dependent primary cilia assembly pathway appeared to function independently of Odf2. Consistently, Cenexin1, but not Odf2, interacted with GTP-loaded Rab8a, localized to the distal/subdistal appendages of basal bodies, and facilitated the recruitment of Chibby, a centriolar component that is important for proper ciliogenesis. Taken together, our results suggest that Cenexin1 plays a critical role in ciliogenesis through its C-terminal extension that confers a unique ability to mediate primary cilia assembly. The presence of multiple splicing variants hints that the function of Odf2 is diversified in such a way that each variant has a distinct role in the complex cellular and developmental processes.
Keywords: Cenexin1; Chibby; Odf2; Rab8a; ciliogenesis; primary cilia.
Figures

Comment in
-
Cenexin1 and Odf2: splice variants with diverged cilium functions.Cell Cycle. 2013 Mar 15;12(6):869. doi: 10.4161/cc.24152. Epub 2013 Mar 7. Cell Cycle. 2013. PMID: 23470458 Free PMC article. No abstract available.
-
One among many: ODF2 isoform 9, a.k.a. Cenexin-1, is required for ciliogenesis.Cell Cycle. 2013 Apr 1;12(7):1021. doi: 10.4161/cc.24330. Epub 2013 Mar 19. Cell Cycle. 2013. PMID: 23511168 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
