Redox artifacts in electrophysiological recordings
- PMID: 23344161
- PMCID: PMC3625719
- DOI: 10.1152/ajpcell.00318.2012
Redox artifacts in electrophysiological recordings
Abstract
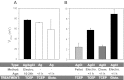
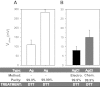
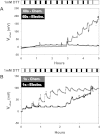
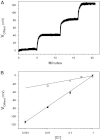
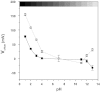
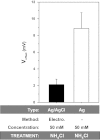
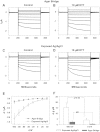
Electrophysiological techniques make use of Ag/AgCl electrodes that are in direct contact with cells or bath. In the bath, electrodes are exposed to numerous experimental conditions and chemical reagents that can modify electrode voltage. We examined voltage offsets created in Ag/AgCl electrodes by exposure to redox reagents used in electrophysiological studies. Voltage offsets were measured in reference to an electrode separated from the solution by an agar bridge. The reducing reagents Tris-2-carboxyethly-phosphine, dithiothreitol (DTT), and glutathione, as well as the oxidizing agent H(2)O(2) used at experimentally relevant concentrations reacted with Ag in the electrodes to produce voltage offsets. Chloride ions and strong acids and bases produced offsets at millimolar concentrations. Electrolytic depletion of the AgCl layer, to replicate voltage clamp and sustained use, resulted in increased sensitivity to flow and DTT. Offsets were sensitive to electrode silver purity and to the amount and method of chloride deposition. For example, exposure to 10 μM DTT produced a voltage offset between 10 and 284 mV depending on the chloride deposition method. Currents generated by these offsets are significant and dependent on membrane conductance and by extension the expression of ion channels and may therefore appear to be biological in origin. These data demonstrate a new source of artifacts in electrophysiological recordings that can affect measurements obtained from a variety of experimental techniques from patch clamp to two-electrode voltage clamp.
Figures


References
-
- Adeva MM, Souto G, Blanco N, Donapetry C. Ammonium metabolism in humans. Metabolism 61: 1495–1511, 2012 - PubMed
-
- Alesutan I, Daryadel A, Mohebbi N, Pelzl L, Leibrock C, Voelkl J, Bourgeois S, Dossena S, Nofziger C, Paulmichl M, Wagner CA, Lang F. Impact of bicarbonate, ammonium chloride, and acetazolamide on hepatic and renal SLC26A4 expression. Cell Physiol Biochem 28: 553–558, 2011 - PubMed
-
- Asami M, Kosaka K, Kunikane S. Bromate, chlorate, chlorite and perchlorate in sodium hypochlorite solution used in water supply. J Water Supply 58: 107–115, 2009
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

