Chemical constituents of three Allium species from Romania
- PMID: 23344191
- PMCID: PMC6270514
- DOI: 10.3390/molecules18010114
Chemical constituents of three Allium species from Romania
Abstract
The aim of this work was to study the chemical composition of Allium obliquum L., A. senescens L. subsp. montanum (Fries) Holub, and A. schoenoprasum L. subsp. schoenoprasum. Sulphur-containing compounds analysis was performed by an LC-MS method, the identification and quantification of polyphenolic compounds through a HPLC-UV-MS method, and the presence of five sterols was simultaneously assessed by HPLC-MS-MS. Alliin was identified only in A. obliquum and A. senescens subsp. montanum extracts, whilst allicin was present in all extracts, with higher amounts in A. schoenoprasum and A. obliquum. The pattern of phenol carboxylic acids shows the presence of p-coumaric and ferulic acids in all species. Isoquercitrin was identified in A. obliquum and A. schoenoprasum, and rutin in A. senescens subsp. montanum and A. schoenoprasum. Luteolin and apigenin were identified only in A. obliquum. All three species contain glycosides of kaempferol and quercetol. β-Sitosterol and campesterol were identified in all species. The results obtained showed significant differences in the composition of the three Allium species.
Figures
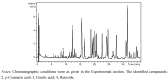
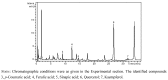

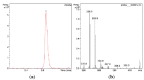

Similar articles
-
Determination of some polyphenolic compounds from Allium species by HPLC-UV-MS.Nat Prod Res. 2010 Sep;24(14):1318-24. doi: 10.1080/14786410903309484. Nat Prod Res. 2010. PMID: 20419559
-
Occurrence and taxonomic significance of cysteine sulphoxides in the genus Allium L. (Alliaceae).Phytochemistry. 2006 Jun;67(11):1127-35. doi: 10.1016/j.phytochem.2006.03.006. Epub 2006 Apr 19. Phytochemistry. 2006. PMID: 16626766
-
Allium chemistry: identification of organosulfur compounds in ramp (Allium tricoccum) homogenates.Phytochemistry. 1998 Sep;49(2):359-64. doi: 10.1016/s0031-9422(98)00191-5. Phytochemistry. 1998. PMID: 9747536
-
Determination of total polyphenolic compounds and flavonoids in Matricaria chamomella flowers.Pak J Pharm Sci. 2019 Sep;32(5):2163-2165. Pak J Pharm Sci. 2019. PMID: 31813883
-
Antioxidant Properties and Structure-Antioxidant Activity Relationship of Allium Species Leaves.Molecules. 2021 Nov 26;26(23):7175. doi: 10.3390/molecules26237175. Molecules. 2021. PMID: 34885755 Free PMC article. Review.
Cited by
-
The Effects of Allium sativum L., Artemisia absinthium L., Cucurbita pepo L., Coriandrum sativum L., Satureja hortensis L. and Calendula officinalis L. on the Embryogenesis of Ascaris suum Eggs during an In Vitro Experimental Study.Pathogens. 2022 Sep 19;11(9):1065. doi: 10.3390/pathogens11091065. Pathogens. 2022. PMID: 36145497 Free PMC article.
-
Antimicrobial and antioxidant activities and phenolic profile of Eucalyptus globulus Labill. and Corymbia ficifolia (F. Muell.) K.D. Hill & L.A.S. Johnson leaves.Molecules. 2015 Mar 16;20(3):4720-34. doi: 10.3390/molecules20034720. Molecules. 2015. PMID: 25786160 Free PMC article.
-
Enhanced Recovery of Antioxidant Compounds from Hazelnut (Corylus avellana L.) Involucre Based on Extraction Optimization: Phytochemical Profile and Biological Activities.Antioxidants (Basel). 2019 Oct 8;8(10):460. doi: 10.3390/antiox8100460. Antioxidants (Basel). 2019. PMID: 31597384 Free PMC article.
-
In Vitro Cytoprotective Effects and Antioxidant Capacity of Phenolic Compounds from the Leaves of Swietenia macrophylla.Molecules. 2015 Oct 16;20(10):18777-88. doi: 10.3390/molecules201018777. Molecules. 2015. PMID: 26501245 Free PMC article.
-
Garlic: Much More Than a Common Spice.Foods. 2020 Oct 26;9(11):1544. doi: 10.3390/foods9111544. Foods. 2020. PMID: 33114554 Free PMC article.
References
-
- Pârvu M., Pârvu A.E., Rosca-Casian O., Vlase L., Groza G. Antifungal activity of Allium obliquum. J. Med. Plants Res. 2010;4:138–141.
-
- Newall C.A., Anderson A.A., Phillipson J.D. Herbal Medicines: A Guide for Health-Care Professionals. The Pharmaceutical Press; London, UK: 1996. pp. 129–133.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

