Development of a 2',4'-BNA/LNA-based siRNA for Dyslipidemia and Assessment of the Effects of Its Chemical Modifications In Vivo
- PMID: 23344237
- PMCID: PMC3464879
- DOI: 10.1038/mtna.2012.32
Development of a 2',4'-BNA/LNA-based siRNA for Dyslipidemia and Assessment of the Effects of Its Chemical Modifications In Vivo
Abstract
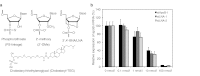
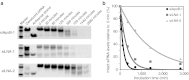
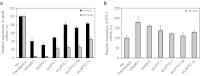
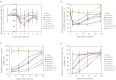
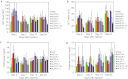
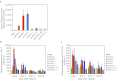
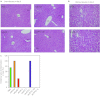
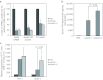
Recent advances in RNA interference (RNAi)-based drug development have partially allowed systemic administration of these agents in vivo with promising therapeutic effects. However, before chemically modified small-interfering RNAs (siRNAs) can be applied clinically, their in vivo effects should be thoroughly assessed. And while many studies have assessed the effects of chemically modified siRNAs in vitro, there has been no comprehensive assessment of their effects in vivo. Here, we aimed to elucidate the effects of administering chemically modified siRNAs in vivo and to propose a 2',4'-bridged nucleic acid (BNA)/locked nucleic acid (LNA)-based siRNA candidate for dyslipidemia. A potentially therapeutic siRNA, siL2PT-1M, was modified with phosphorothioate (PS) and 2',4'-BNA/LNA in its sense strand and with 2'-methoxy (2'-OMe) nucleotides in its immunostimulatory motif; administration of siL2PT-1M resulted in sustained reductions in serum total cholesterol (TC) (24 days) and a concomitant apolipoprotein B (apoB) mRNA reduction in liver without adverse effects. The 2',4'-BNA/LNA modification in the sense strand was greatly augmented the duration of the RNAi effect, whereas cholesterol conjugation shortened the duration. Cholesterol-conjugated immunostimulatory siRNA (isRNA) induced higher serum interferon-α (IFN-α) levels than did nonmodified isRNA, indicating that the immune reaction was facilitated by cholesterol conjugation. Our results indicated that modification of the adenosine residues complementary to the immunostimulatory motif and of central 5'-UG-3' in the sense strand would ameliorate the negative immune response.Molecular Therapy - Nucleic Acids (2012) 1, e45; doi:10.1038/mtna.2012.32; published online 18 September 2012.
Figures
References
-
- Hannon GJ. RNA interference. Nature. 2002;418:244–251. - PubMed
-
- McManus MT., and, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3:737–747. - PubMed
-
- White PJ. Barriers to successful delivery of short interfering RNA after systemic administration. Clin Exp Pharmacol Physiol. 2008;35:1371–1376. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

