Dystrophin deficiency leads to disturbance of LAMP1-vesicle-associated protein secretion
- PMID: 23344255
- PMCID: PMC11113779
- DOI: 10.1007/s00018-012-1248-2
Dystrophin deficiency leads to disturbance of LAMP1-vesicle-associated protein secretion
Abstract
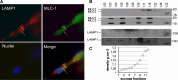
Duchenne muscular dystrophy results from loss of the protein dystrophin, which links the intracellular cytoskeletal network with the extracellular matrix, but deficiency in this function does not fully explain the onset or progression of the disease. While some intracellular events involved in the degeneration of dystrophin-deficient muscle fibers have been well characterized, changes in their secretory profile are undescribed. To analyze the secretome profile of mdx myotubes independently of myonecrosis, we labeled the proteins of mdx and wild-type myotubes with stable isotope-labeled amino acids (SILAC), finding marked enrichment of vesicular markers in the mdx secretome. These included the lysosomal-associated membrane protein, LAMP1, that co-localized in vesicles with an over-secreted cytoskeletal protein, myosin light chain 1. These LAMP1/MLC1-3-positive vesicles accumulated in the cytosol of mdx myotubes and were secreted into the culture medium in a range of abnormal densities. Restitution of dystrophin expression, by exon skipping, to some 30 % of the control value, partially normalized the secretome profile and the excess LAMP1 accumulation. Together, our results suggest that a lack of dystrophin leads to a general dysregulation of vesicle trafficking. We hypothesize that disturbance of the export of proteins through vesicles occurs before, and then concurrently with, the myonecrotic cascade and contributes chronically to the pathophysiology of DMD, thereby presenting us with a range of new potential therapeutic targets.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures






References
-
- Engel A, Arahata K, Biesecker G. Mechanisms of muscle fiber destruction. In: Serratrice G, editor. Neuromuscular diseases. New York: Raven Press; 1984. pp. 137–141.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

