B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma
- PMID: 23344265
- PMCID: PMC3630268
- DOI: 10.1158/1078-0432.CCR-12-2422
B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma
Abstract
Purpose: Multiple myeloma is a usually incurable malignancy of plasma cells. New therapies are urgently needed for multiple myeloma. Adoptive transfer of chimeric antigen receptor (CAR)-expressing T cells is a promising new therapy for hematologic malignancies, but an ideal target antigen for CAR-expressing T-cell therapies for multiple myeloma has not been identified. B-cell maturation antigen (BCMA) is a protein that has been reported to be selectively expressed by B-lineage cells including multiple myeloma cells. Our goal was to determine if BCMA is a suitable target for CAR-expressing T cells.
Experimental design: We conducted an assessment of BCMA expression in normal human tissues and multiple myeloma cells by flow cytometry, quantitative PCR, and immunohistochemistry. We designed and tested novel anti-BCMA CARs.
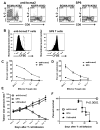
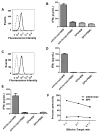
Results: BCMA had a restricted RNA expression pattern. Except for expression in plasma cells, BCMA protein was not detected in normal human tissues. BCMA was not detected on primary human CD34(+) hematopoietic cells. We detected uniform BCMA cell-surface expression on primary multiple myeloma cells from five of five patients. We designed the first anti-BCMA CARs to be reported and we transduced T cells with lentiviral vectors encoding these CARs. The CARs gave T cells the ability to specifically recognize BCMA. The anti-BCMA-CAR-transduced T cells exhibited BCMA-specific functions including cytokine production, proliferation, cytotoxicity, and in vivo tumor eradication. Importantly, anti-BCMA-CAR-transduced T cells recognized and killed primary multiple myeloma cells.
Conclusions: BCMA is a suitable target for CAR-expressing T cells, and adoptive transfer of anti-BCMA-CAR-expressing T cells is a promising new strategy for treating multiple myeloma.
Conflict of interest statement
A patent application covering anti-BCMA chimeric antigen receptors was filed by the NIH. JNK is the inventor on this patent. There are no other conflicts of interest.
Figures



Comment in
-
Zoom Zoom: racing CARs for multiple myeloma.Clin Cancer Res. 2013 Apr 15;19(8):1917-9. doi: 10.1158/1078-0432.CCR-13-0168. Epub 2013 Feb 26. Clin Cancer Res. 2013. PMID: 23444214 Free PMC article.
References
-
- Lonial S, Mitsiades CS, Richardson PG. Treatment options for relapsed and refractory multiple myeloma. Clinical Cancer Research. 2011;17:1264–77. - PubMed
-
- Palumbo A, Anderson K. Multiple myeloma. New England Journal of Medicine. 2011;364:1046–60. - PubMed
-
- Salit RB, Bishop MR. Reduced-intensity allogeneic hematopoietic stem cell transplantation for multiple myeloma: A concise review. Clinical Lymphoma, Myeloma and Leukemia. 2011;11:247–52. - PubMed
-
- Richardson PG, Lonial S, Jakubowiak AJ, Harousseau JL, Anderson KC. Monoclonal antibodies in the treatment of multiple myeloma. British Journal of Haematology. 2011;154:745–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

