High viral load and elevated angiogenic markers associated with increased risk of preeclampsia among women initiating highly active antiretroviral therapy in pregnancy in the Mma Bana study, Botswana
- PMID: 23344545
- PMCID: PMC3683097
- DOI: 10.1097/QAI.0b013e318286d77e
High viral load and elevated angiogenic markers associated with increased risk of preeclampsia among women initiating highly active antiretroviral therapy in pregnancy in the Mma Bana study, Botswana
Abstract
Background: Risk factors associated with preeclampsia in HIV-infected women remain largely unknown. Systemic angiogenic imbalance contributes to preeclampsia in HIV-uninfected women, but changes in angiogenic markers after highly active antiretroviral therapy (HAART) initiation have not been studied.
Methods: The Mma Bana study randomized 560 HIV-infected, HAART-naive pregnant women with CD4 counts ≥ 200 cells per cubic millimeter between 26 and 34 weeks gestation to lopinavir/ritonavir/zidovudine/lamivudine or abacavir/zidovudine/lamivudine. Another 170 participants with CD4 counts less than 200 cells per cubic millimeter initiated nevirapine/zidovudine/lamivudine between 18 and 34 weeks gestation. Characteristics of 11 women who developed preeclampsia were compared with the remaining 722 Mma Bana participants who delivered using logistic regression. Plasma samples drawn at HAART initiation and 1 month later from 60 women without preeclampsia and at HAART initiation for all 11 preeclamptic women were assayed for placental growth factor (PlGF) and soluble FMS toll-like tyrosine kinase-1 (sFlt-1).
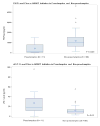
Results: Pre-HAART viral load greater than 100,000 copies per milliliter was associated with preeclampsia (odds ratio: 5.8, 95% confidence interval: 1.8 to 19.4, P = 0.004). Median pre-HAART PlGF level was lower and sFlt-1 was higher in women who developed preeclampsia vs those who did not (130 vs 992 pg/mL, P = 0.001; 17.5 vs 9.4 pg/mL, P = 0.03, respectively). In multivariate analysis, PlGF and viral load remained significantly associated with preeclampsia. No significant changes in angiogenic factors were noted after 1 month of HAART treatment among non-preeclamptic women.
Conclusions: Pre-HAART viral load greater than 100,000 copies per milliliter and PlGF predicted preeclampsia among women starting HAART in pregnancy. Among non-preeclamptic women, HAART treatment did not significantly alter levels of PlGF or sFlt-1 after 1 month of treatment.
Conflict of interest statement
MH has served as a paid DSMB member for Beohringer Ingelheim, Pfizer, Tibotec and Medicines Development.
References
-
- Dolea C, AbouZahr C. Global burden of hypertensive disorders of pregnancy in the year 2000: Evidence and Information for Policy (EIP) World Health Organization; Geneva: Jul, 2003. [Accessed 8-Oct-11]. http://www.who.int/healthinfo/statistics/bod_hypertensivedisordersofpreg....
-
- Villar J, Say L, Shennan A, et al. Methodological and technical issues related to the diagnosis, screening, prevention, and treatment of preeclampsia and eclampsia. International Journal of Gynecology and Obstetrics. 2004;85(Suppl 1):S28–S41. - PubMed
-
- Conde-Agudelo A, Villar J, Lindheimer M. Maternal infection and risk of preeclampsia: Systematic review and metaanalysis. American Journal of Obstetrics & Gynecology. 2008;198(1):7–22. - PubMed
-
- Firoz T, Sanghvi H, Merialdi M, et al. Preeclampsia in low and middle income countries. Best Practices and Research Clinical Obstetrics and Gynaecology. 2011;25:537–548. - PubMed
-
- Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systemic review. Lancet. 2006;367:1066–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous