Pseudotyped retroviruses for infecting axolotl in vivo and in vitro
- PMID: 23344705
- PMCID: PMC3583047
- DOI: 10.1242/dev.087734
Pseudotyped retroviruses for infecting axolotl in vivo and in vitro
Abstract
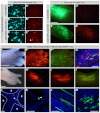
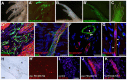
Axolotls are poised to become the premiere model system for studying vertebrate appendage regeneration. However, very few molecular tools exist for studying crucial cell lineage relationships over regeneration or for robust and sustained misexpression of genetic elements to test their function. Furthermore, targeting specific cell types will be necessary to understand how regeneration of the diverse tissues within the limb is accomplished. We report that pseudotyped, replication-incompetent retroviruses can be used in axolotls to permanently express markers or genetic elements for functional study. These viruses, when modified by changing their coat protein, can infect axolotl cells only when they have been experimentally manipulated to express the receptor for that coat protein, thus allowing for the possibility of targeting specific cell types. Using viral vectors, we have found that progenitor populations for many different cell types within the blastema are present at all stages of limb regeneration, although their relative proportions change with time.
Figures




Similar articles
-
Pseudotyped retroviruses for infecting axolotl.Methods Mol Biol. 2015;1290:127-40. doi: 10.1007/978-1-4939-2495-0_10. Methods Mol Biol. 2015. PMID: 25740482
-
Pseudotyped baculovirus is an effective gene expression tool for studying molecular function during axolotl limb regeneration.Dev Biol. 2018 Jan 15;433(2):262-275. doi: 10.1016/j.ydbio.2017.10.008. Epub 2017 Nov 30. Dev Biol. 2018. PMID: 29198566
-
Transgenesis in axolotl (Ambystoma mexicanum).Methods Mol Biol. 2015;1290:269-77. doi: 10.1007/978-1-4939-2495-0_21. Methods Mol Biol. 2015. PMID: 25740493
-
Identification of immune and non-immune cells in regenerating axolotl limbs by single-cell sequencing.Exp Cell Res. 2020 Sep 15;394(2):112149. doi: 10.1016/j.yexcr.2020.112149. Epub 2020 Jun 18. Exp Cell Res. 2020. PMID: 32562784 Free PMC article. Review.
-
No false start for novel pseudotyped vectors.Curr Opin Biotechnol. 2002 Oct;13(5):437-42. doi: 10.1016/s0958-1669(02)00374-9. Curr Opin Biotechnol. 2002. PMID: 12459334 Review.
Cited by
-
Transcriptomic landscape of the blastema niche in regenerating adult axolotl limbs at single-cell resolution.Nat Commun. 2018 Dec 4;9(1):5153. doi: 10.1038/s41467-018-07604-0. Nat Commun. 2018. PMID: 30514844 Free PMC article.
-
CRISPR-mediated genomic deletion of Sox2 in the axolotl shows a requirement in spinal cord neural stem cell amplification during tail regeneration.Stem Cell Reports. 2014 Sep 9;3(3):444-59. doi: 10.1016/j.stemcr.2014.06.018. Epub 2014 Aug 7. Stem Cell Reports. 2014. PMID: 25241743 Free PMC article.
-
Precise control of ion channel and gap junction expression is required for patterning of the regenerating axolotl limb.Int J Dev Biol. 2020;64(10-11-12):485-494. doi: 10.1387/ijdb.200114jw. Int J Dev Biol. 2020. PMID: 33200809 Free PMC article.
-
VEGF signaling promotes blastema growth and proliferation of vascular and non-vascular cells during axolotl limb regeneration.Dev Biol. 2025 Sep;525:206-215. doi: 10.1016/j.ydbio.2025.05.030. Epub 2025 Jun 5. Dev Biol. 2025. PMID: 40480306
-
Eya2 promotes cell cycle progression by regulating DNA damage response during vertebrate limb regeneration.Elife. 2020 Mar 6;9:e51217. doi: 10.7554/eLife.51217. Elife. 2020. PMID: 32142407 Free PMC article.
References
-
- Berg D. A., Kirkham M., Beljajeva A., Knapp D., Habermann B., Ryge J., Tanaka E. M., Simon A. (2010). Efficient regeneration by activation of neurogenesis in homeostatically quiescent regions of the adult vertebrate brain. Development 137, 4127–4134 - PubMed
-
- Brockes J. P., Kumar A. (2005). Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science 310, 1919–1923 - PubMed
-
- Burns J. C., Matsubara T., Lozinski G., Yee J. K., Friedmann T., Washabaugh C. H., Tsonis P. A. (1994). Pantropic retroviral vector-mediated gene transfer, integration, and expression in cultured newt limb cells. Dev. Biol. 165, 285–289 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

