A randomized, double-blind, placebo-controlled study to evaluate the effect of repeated oral doses of pazopanib on cardiac conduction in patients with solid tumors
- PMID: 23344712
- PMCID: PMC3899892
- DOI: 10.1007/s00280-012-2030-8
A randomized, double-blind, placebo-controlled study to evaluate the effect of repeated oral doses of pazopanib on cardiac conduction in patients with solid tumors
Abstract
Purpose: As tyrosine kinase inhibitors have been associated with cardiotoxicity, we evaluated the effect of pazopanib, an inhibitor of vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and c-Kit, on electrocardiographic parameters in patients with cancer.
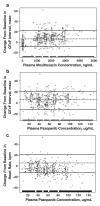
Methods: This double-blind, placebo-controlled, parallel-group study randomized patients (N = 96) to moxifloxacin (positive control) or placebo on Day 1 followed by pazopanib or placebo 800 mg/day (fasted) on Days 2-8 and 1,600 mg (with food) on Day 9. Treatment effects were evaluated by baseline-adjusted, time-matched, serial Holter electrocardiograms.
Results: Sixty-five patients were evaluable for preplanned analyses. On Day 1, the maximum mean difference in baseline-adjusted, time-matched Fridericia-corrected QT (QTcF) interval in moxifloxacin-treated patients versus placebo was 10.6 ms (90% confidence interval [CI]: 4.2, 17.0). The administration scheme increased plasma pazopanib concentrations approximately 1.3- to 1.4-fold versus the recommended 800 mg once-daily dose. Pazopanib caused clinically significant increases from baseline in blood pressure, an anticipated class effect, and an unexpected reduction in heart rate from baseline that correlated with pazopanib exposure. On Day 9, the maximum mean difference in baseline-adjusted, time-matched QTcF interval in pazopanib-treated patients versus placebo was 4.4 ms (90% CI: -2.4, 11.2). Mixed-effects modeling indicated no significant concentration-dependent effect of pazopanib or its metabolites on QTcF interval.
Conclusions: Pazopanib as administered in this study achieved supratherapeutic concentrations, produced a concentration-dependent decrease in heart rate, and caused a small, concentration-independent prolongation of the QTcF interval.
Figures



References
-
- Hutson TE, Davis ID, Machiels JP, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010;28:475–480. - PubMed
-
- Sonpavde G, Hutson TE, Sternberg CN. Pazopanib, a potent orally administered small-molecule multitargeted tyrosine kinase inhibitor for renal cell carcinoma. Expert Opin Investig Drugs. 2008;17:253–261. - PubMed
-
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. - PubMed
-
- Australian Government Department of Health and Ageing. Drugs designated as orphan drugs; Pazopanib (PATORMA): [Accessed 23 July 2012]. 2010. http://www.tga.gov.au/industry/pm-orphan-drugs.htm.
-
- Instituto de Salud Publica de Chile [Accessed 23 July 2012];Votrient coated tablets [product registration] 2010 http://200.68.11.21/RegistrosISP/fiFichaProducto.asp?RegistroISP=F-18018/10.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

