Optimized in vivo transfer of small interfering RNA targeting dermal tissue using in vivo surface electroporation
- PMID: 23344722
- PMCID: PMC3381603
- DOI: 10.1038/mtna.2012.1
Optimized in vivo transfer of small interfering RNA targeting dermal tissue using in vivo surface electroporation
Abstract
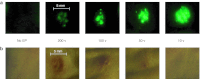
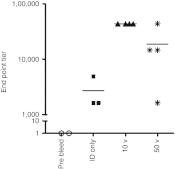
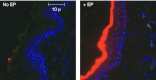
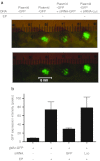
Electroporation (EP) of mammalian tissue is a technique that has been used successfully in the clinic for the delivery of genetic-based vaccines in the form of DNA plasmids. There is great interest in platforms which efficiently deliver RNA molecules such as messenger RNA and small interfering RNA (siRNA) to mammalian tissue. However, the in vivo delivery of RNA enhanced by EP has not been extensively characterized. This paper details the optimization of electrical parameters for a novel low-voltage EP method to deliver oligonucleotides (both DNA and RNA) to dermal tissue in vivo. Initially, the electrical parameters were optimized for dermal delivery of plasmid DNA encoding green fluorescent protein (GFP) using this novel surface dermal EP device. While all investigated parameters resulted in visible transfection, voltage parameters in the 10 V range elicited the most robust signal. The parameters optimized for DNA, were then assessed for translation of successful electrotransfer of siRNA into dermal tissue. Robust tagged-siRNA transfection in skin was detected. We then assessed whether these parameters translated to successful transfer of siRNA resulting in gene knockdown in vivo. Using a reporter gene construct encoding GFP and tagged siRNA targeting the GFP message, we show simultaneous transfection of the siRNA to the skin via EP and the concomitant knockdown of the reporter gene signal. The siRNA delivery was accomplished with no evidence of injection site inflammation or local tissue damage. The minimally invasive low-voltage EP method is thus capable of efficiently delivering both DNA and RNA molecules to dermal tissue in a tolerable manner.
Figures


References
-
- Higuchi Y, Kawakami S., and, Hashida M. Strategies for in vivo delivery of siRNAs: recent progress. BioDrugs. 2010;24:195–205. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

