Intracerebral Infusion of Antisense Oligonucleotides Into Prion-infected Mice
- PMID: 23344724
- PMCID: PMC3381600
- DOI: 10.1038/mtna.2011.6
Intracerebral Infusion of Antisense Oligonucleotides Into Prion-infected Mice
Abstract
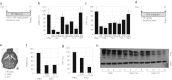
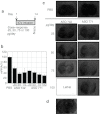
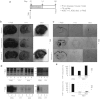
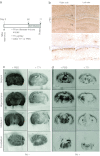
Mice deficient for the cellular prion protein (PrP(C)) do not develop prion disease; accordingly, gene-based strategies to diminish PrP(C) expression are of interest. We synthesized a series of chemically modified antisense oligonucleotides (ASOs) targeted against mouse Prnp messenger RNA (mRNA) and identified those that were most effective in decreasing PrP(C) expression. Those ASOs were also evaluated in scrapie-infected cultured cells (ScN2a) for their efficacy in diminishing the levels of the disease-causing prion protein (PrP(Sc)). When the optimal ASO was infused intracerebrally into FVB mice over a 14-day period beginning 1 day after infection with the Rocky Mountain Laboratory (RML) strain of mouse prions, a prolongation of the incubation period of almost 2 months was observed. Whether ASOs can be used to develop an effective therapy for patients dying of Creutzfeldt-Jakob disease remains to be established.
Figures



References
-
- Tilly G, Chapuis J, Vilette D, Laude H., and, Vilotte JL. Efficient and specific down-regulation of prion protein expression by RNAi. Biochem Biophys Res Commun. 2003;305:548–551. - PubMed
-
- Daude N, Marella M., and, Chabry J. Specific inhibition of pathological prion protein accumulation by small interfering RNAs. J Cell Sci. 2003;116 Pt 13:2775–2779. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

