Good Cascade Impactor Practice (GCIP) and considerations for "in-use" specifications
- PMID: 23344853
- PMCID: PMC3581645
- DOI: 10.1208/s12249-012-9905-1
Good Cascade Impactor Practice (GCIP) and considerations for "in-use" specifications
Abstract
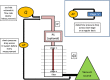
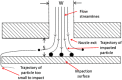
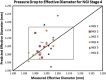
The multi-stage cascade impactor (CI) is widely used to determine aerodynamic particle size distributions (APSDs) of orally inhaled products. Its size-fractionating capability depends primarily on the size of nozzles of each stage. Good Cascade Impactor Practice (GCIP) requires that these critical dimensions are linked to the accuracy of the APSD measurement based on the aerodynamic diameter size scale. Effective diameter (Deff) is the critical dimension describing any nozzle array, as it is directly related to stage cut-point size (d50). d50 can in turn be determined by calibration using particles of known aerodynamic diameter, providing traceability to the international length standard. Movements in Deff within manufacturer tolerances for compendial CIs result in the worst case in shifts in d50 of <±10%. Stage mensuration therefore provides satisfactory control of measurement accuracy. The accurate relationship of Deff to d50 requires the CI system to be leak-free, which can be checked by sealing the apparatus at the entry to the induction port and isolating it from the vacuum source and measuring the rate of pressure rise before each use. Mensuration takes place on an infrequent basis compared with the typical interval between individual APSD determinations. Measurement of stage flow resistance (pressure drop; ΔPstage) could enable the user to know that the CI stages are fit for use before every APSD measurement, by yielding an accurate measure of Deff. However, more data are needed to assess the effects of wear and blockage before this approach can be advocated as part of GCIP.
Figures

References
-
- Newman SP, Chan H-K. In vitro/in vivo comparisons in pulmonary drug delivery. J Aerosol Med. 2008;21(1):1–8. - PubMed
-
- European Directorate for Quality in Medicines and HealthCare (EDQM). Monograph 2.9.18: European Pharmacopeia, Preparations for inhalation: aerodynamic assessment of fine particles, Council of Europe, 67075 Strasbourg, France, 2012; Ph.Eur.7.5 [USB 7th ed. (7.52012)].
-
- United States Pharmacopeial Convention. Chapter 601: Aerosols, Nasal Sprays, Metered-Dose Inhalers and Dry Powder Inhalers. Rockville, MD, USA, 2012; USP 35-NF 30.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

