Stimulus change detection in phasic auditory units in the frog midbrain: frequency and ear specific adaptation
- PMID: 23344947
- PMCID: PMC3700364
- DOI: 10.1007/s00359-013-0794-x
Stimulus change detection in phasic auditory units in the frog midbrain: frequency and ear specific adaptation
Abstract
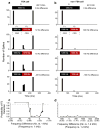
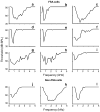
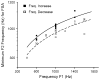
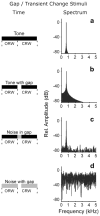
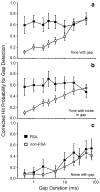
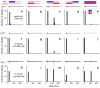
Neural adaptation, a reduction in the response to a maintained stimulus, is an important mechanism for detecting stimulus change. Contributing to change detection is the fact that adaptation is often stimulus specific: adaptation to a particular stimulus reduces excitability to a specific subset of stimuli, while the ability to respond to other stimuli is unaffected. Phasic cells (e.g., cells responding to stimulus onset) are good candidates for detecting the most rapid changes in natural auditory scenes, as they exhibit fast and complete adaptation to an initial stimulus presentation. We made recordings of single phasic auditory units in the frog midbrain to determine if adaptation was specific to stimulus frequency and ear of input. In response to an instantaneous frequency step in a tone, 28% of phasic cells exhibited frequency specific adaptation based on a relative frequency change (delta-f=±16%). Frequency specific adaptation was not limited to frequency steps, however, as adaptation was also overcome during continuous frequency modulated stimuli and in response to spectral transients interrupting tones. The results suggest that adaptation is separated for peripheral (e.g., frequency) channels. This was tested directly using dichotic stimuli. In 45% of binaural phasic units, adaptation was ear specific: adaptation to stimulation of one ear did not affect responses to stimulation of the other ear. Thus, adaptation exhibited specificity for stimulus frequency and lateralization at the level of the midbrain. This mechanism could be employed to detect rapid stimulus change within and between sound sources in complex acoustic environments.
Figures





References
-
- Bass AH, Rose GJ, Pritz MB. Auditory midbrain of fish, amphibians and reptiles: model systems for understanding auditory function. In: Winer JA, Schreiner CE, editors. The inferior colliculus. Springer; New York: 2005. pp. 459–492.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

