Advanced techniques for constrained internal coordinate molecular dynamics
- PMID: 23345138
- PMCID: PMC3835462
- DOI: 10.1002/jcc.23200
Advanced techniques for constrained internal coordinate molecular dynamics
Abstract
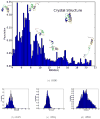
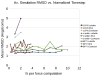
Internal coordinate molecular dynamics (ICMD) methods provide a more natural description of a protein by using bond, angle, and torsional coordinates instead of a Cartesian coordinate representation. Freezing high-frequency bonds and angles in the ICMD model gives rise to constrained ICMD (CICMD) models. There are several theoretical aspects that need to be developed to make the CICMD method robust and widely usable. In this article, we have designed a new framework for (1) initializing velocities for nonindependent CICMD coordinates, (2) efficient computation of center of mass velocity during CICMD simulations, (3) using advanced integrators such as Runge-Kutta, Lobatto, and adaptive CVODE for CICMD simulations, and (4) cancelling out the "flying ice cube effect" that sometimes arises in Nosé-Hoover dynamics. The Generalized Newton-Euler Inverse Mass Operator (GNEIMO) method is an implementation of a CICMD method that we have developed to study protein dynamics. GNEIMO allows for a hierarchy of coarse-grained simulation models based on the ability to rigidly constrain any group of atoms. In this article, we perform tests on the Lobatto and Runge-Kutta integrators to determine optimal simulation parameters. We also implement an adaptive coarse-graining tool using the GNEIMO Python interface. This tool enables the secondary structure-guided "freezing and thawing" of degrees of freedom in the molecule on the fly during molecular dynamics simulations and is shown to fold four proteins to their native topologies. With these advancements, we envision the use of the GNEIMO method in protein structure prediction, structure refinement, and in studying domain motion.
Copyright © 2013 Wiley Periodicals, Inc.
Figures






References
-
- Mazur A, RA A. J Biomol Struct Dyn. 1989;6:815. - PubMed
-
- Mazur AK, Dorofeev VE, Abagyan RA. J Comput Phys. 1991;92:261.
-
- Jain A, Vaidehi N, Rodriguez G. J Comput Phys. 1993;106:258.
-
- Chen J, Im W, Brooks CL., III J Comput Chem. 2005;26:1565. - PubMed
-
- Gibson KD, Scheraga HA. J Comput Chem. 1990;11:468.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

