Ataxia with cerebellar lesions in mice expressing chimeric PrP-Dpl protein
- PMID: 23345215
- PMCID: PMC6618747
- DOI: 10.1523/JNEUROSCI.2231-12.2013
Ataxia with cerebellar lesions in mice expressing chimeric PrP-Dpl protein
Abstract
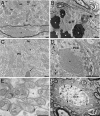
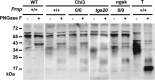
Mutations within the central region of prion protein (PrP) have been shown to be associated with severe neurotoxic activity similar to that observed with Dpl, a PrP-like protein. To further investigate this neurotoxic effect, we generated lines of transgenic (Tg) mice expressing three different chimeric PrP-Dpl proteins. Chi1 (amino acids 1-57 of Dpl replaced by amino acids 1-125 of PrP) and Chi2 (amino acids 1-66 of Dpl replaced by amino acids 1-134 of PrP) abrogated the pathogenicity of Dpl indicating that the presence of a N-terminal domain of PrP (23-134) reduced the toxicity of Dpl, as reported. However, when the amino acids 1-24 of Dpl were replaced by amino acids 1-124 of PrP, Chi3 Tg mice, which express the chimeric protein at a very low level, start developing ataxia at the age of 5-7 weeks. This phenotype was not counteracted by a single copy of full-length-PrP(c) but rather by its overexpression, indicating the strong toxicity of the chimeric protein Chi3. Chi3 Tg mice exhibit severe cerebellar atrophy with a significant loss of granule cells. We concluded that aa25 to aa57 of Dpl, which are not present in Chi1 and Chi2 constructs, confer toxicity to the protein. We tested this possibility by using the 25-57 Dpl peptide in primary culture of mouse embryo cortical neurons and found a significant neurotoxic effect. This finding identifies a protein domain that plays a role in mediating Dpl-related toxicity.
Figures




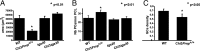



Similar articles
-
Doppel-induced cerebellar degeneration in transgenic mice.Proc Natl Acad Sci U S A. 2001 Dec 18;98(26):15288-93. doi: 10.1073/pnas.251550798. Epub 2001 Dec 4. Proc Natl Acad Sci U S A. 2001. PMID: 11734625 Free PMC article.
-
Deletion of N-terminal residues 23-88 from prion protein (PrP) abrogates the potential to rescue PrP-deficient mice from PrP-like protein/doppel-induced Neurodegeneration.J Biol Chem. 2003 Aug 1;278(31):28944-9. doi: 10.1074/jbc.M303655200. Epub 2003 May 19. J Biol Chem. 2003. PMID: 12759361
-
Transgene-driven expression of the Doppel protein in Purkinje cells causes Purkinje cell degeneration and motor impairment.Proc Natl Acad Sci U S A. 2004 Mar 9;101(10):3644-9. doi: 10.1073/pnas.0308681101. Epub 2004 Mar 8. Proc Natl Acad Sci U S A. 2004. PMID: 15007176 Free PMC article.
-
The prion gene complex encoding PrP(C) and Doppel: insights from mutational analysis.Gene. 2001 Sep 5;275(1):1-18. doi: 10.1016/s0378-1119(01)00627-8. Gene. 2001. PMID: 11574147 Review.
-
Physiological and pathological functions of the prion protein homologue Dpl.Br Med Bull. 2003;66:35-42. doi: 10.1093/bmb/66.1.35. Br Med Bull. 2003. PMID: 14522847 Review.
Cited by
-
Loss of NCB5OR in the cerebellum disturbs iron pathways, potentiates behavioral abnormalities, and exacerbates harmaline-induced tremor in mice.Metab Brain Dis. 2016 Aug;31(4):951-64. doi: 10.1007/s11011-016-9834-x. Epub 2016 May 18. Metab Brain Dis. 2016. PMID: 27188291 Free PMC article.
-
Stearic acid blunts growth-factor signaling via oleoylation of GNAI proteins.Nat Commun. 2021 Jul 28;12(1):4590. doi: 10.1038/s41467-021-24844-9. Nat Commun. 2021. PMID: 34321466 Free PMC article.
References
-
- Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. - PubMed
-
- Al Bersaoui R, Robert I, Lutz Y, Blanc F, Sommermeyer-Leroux G, Shibaguchi H, Aunis D, Fuchs JP. Purkinje-cell degeneration in prion protein-deficient mice is associated with a cerebellum-specific Doppel protein species signature. FEBS Lett. 2005;579:2715–2721. - PubMed
-
- Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C. Normal development and behaviour of mice lacking the neuronal cell- surface PrP protein [see comments] Nature. 1992;356:577–582. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
