Prestimulus oscillatory activity over motor cortex reflects perceptual expectations
- PMID: 23345216
- PMCID: PMC6618755
- DOI: 10.1523/JNEUROSCI.1094-12.2013
Prestimulus oscillatory activity over motor cortex reflects perceptual expectations
Abstract
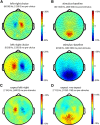
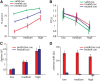
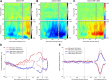
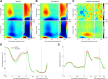
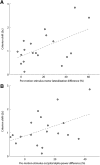
When perceptual decisions are coupled to a specific effector, preparatory motor cortical activity may provide a window into the dynamics of the perceptual choice. Specifically, previous studies have observed a buildup of choice-selective activity in motor regions over time reflecting the integrated sensory evidence provided by visual cortex. Here we ask how this choice-selective motor activity is modified by prior expectation during a visual motion discrimination task. Computational models of decision making formalize decisions as the accumulation of evidence from a starting point to a decision bound. Within this framework, expectation could change the starting point, rate of accumulation, or the decision bound. Using magneto-encephalography in human observers, we specifically tested for changes in the starting point in choice-selective oscillatory activity over motor cortex. Inducing prior expectation about motion direction biased subjects' perceptual judgments as well as the choice-selective motor activity in the 8-30 Hz frequency range before stimulus onset; the individual strength of these behavioral and neural biases were correlated across subjects. In the absence of explicit expectation cues, spontaneous biases in choice-selective activity were evident over motor cortex. These also predicted eventual perceptual choice and were, at least in part, induced by the choice on the previous trial. We conclude that both endogenous and explicitly induced perceptual expectations bias the starting point of decision-related activity, before the accumulation of sensory evidence.
Figures



References
-
- Bastiaansen MC, Knösche TR. Tangential derivative mapping of axial MEG applied to event-related desynchronization research. Clin Neurophysiol. 2000;111:1300–1305. - PubMed
-
- Bogacz R, Brown E, Moehlis J, Holmes P, Cohen JD. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol Rev. 2006;113:700–765. - PubMed
-
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
