Interglomerular lateral inhibition targeted on external tufted cells in the olfactory bulb
- PMID: 23345229
- PMCID: PMC3711647
- DOI: 10.1523/JNEUROSCI.3410-12.2013
Interglomerular lateral inhibition targeted on external tufted cells in the olfactory bulb
Abstract
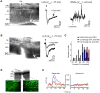
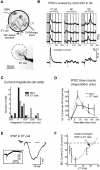
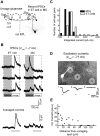
Lateral inhibition between neurons occurs in many different sensory systems, where it can perform such functions as contrast enhancement. In the olfactory bulb, lateral inhibition may occur between odorant receptor-specific glomeruli that are linked anatomically by GABAergic granule cells (GCs) and cells within the glomerular layer, although evidence supporting lateral inhibition at a functional level is modest. Here, we used patch-clamp, imaging, and glutamate uncaging methods in rat olfactory bulb slices to test for the presence of interglomerular lateral inhibition, as well as its underlying mechanisms. We found that a conditioning stimulus applied at one or a small group of glomeruli could suppress stimulus-evoked excitation of output mitral cells (MCs) at another glomerulus for interstimulus intervals of 20-50 ms and glomerular separations of up to 600 μm. The observed lateral inhibition was entirely dependent on circuitry within the glomerular layer, rather than GCs, and it involved GABAergic synaptic inputs that were targeted mainly onto tufted cells, which act as intermediaries in the excitation between olfactory sensory neurons and MCs. The key cell type responsible for mediating lateral interactions between glomeruli were GABAergic short-axon cells. These results suggest a functional segregation of GABAergic cells within the bulb, with one set located in the glomerular layer mediating suppression of MC spiking across glomeruli, and a second set, the GCs, synchronizing different glomeruli.
Figures



References
-
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. - PubMed
-
- Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron. 2008;57:586–598. - PubMed
-
- Bozza TC, Mombaerts P. Olfactory coding: revealing intrinsic representations of odors. Curr Biol. 2001;11:R687–R690. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
