Selective ablation of pillar and deiters' cells severely affects cochlear postnatal development and hearing in mice
- PMID: 23345230
- PMCID: PMC3567488
- DOI: 10.1523/JNEUROSCI.3088-12.2013
Selective ablation of pillar and deiters' cells severely affects cochlear postnatal development and hearing in mice
Abstract
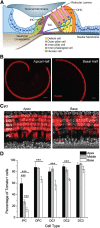
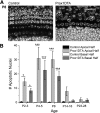
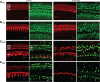
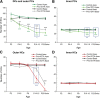
Mammalian auditory hair cells (HCs) are inserted into a well structured environment of supporting cells (SCs) and acellular matrices. It has been proposed that when HCs are irreversibly damaged by noise or ototoxic drugs, surrounding SCs seal the epithelial surface and likely extend the survival of auditory neurons. Because SCs are more resistant to damage than HCs, the effects of primary SC loss on HC survival and hearing have received little attention. We used the Cre/loxP system in mice to specifically ablate pillar cells (PCs) and Deiters' cells (DCs). In Prox1CreER(T2)+/-;Rosa26(DTA/+) (Prox1DTA) mice, Cre-estrogen receptor (CreER) expression is driven by the endogenous Prox1 promoter and, in presence of tamoxifen, removes a stop codon in the Rosa26(DTA/+) allele and induces diphtheria toxin fragment A (DTA) expression. DTA produces cell-autonomous apoptosis. Prox1DTA mice injected with tamoxifen at postnatal days 0 (P0) and P1 show significant DC and outer PC loss at P2-P4, that reaches ∼70% by 1 month. Outer HC loss follows at P14 and is almost complete at 1 month, while inner HCs remain intact. Neural innervation to the outer HCs is disrupted in Prox1DTA mice and auditory brainstem response thresholds in adults are 40-50 dB higher than in controls. The hearing deficit correlates with loss of cochlear amplification. Remarkably, in Prox1DTA mice, the auditory epithelium preserves the ability to seal the reticular lamina and spiral ganglion neuron counts are normal, a key requirement for cochlear implant success. In addition, our results show that cochlear SC pools should be appropriately replenished during HC regeneration strategies.
Figures


Similar articles
-
In vivo proliferation of postmitotic cochlear supporting cells by acute ablation of the retinoblastoma protein in neonatal mice.J Neurosci. 2010 Apr 28;30(17):5927-36. doi: 10.1523/JNEUROSCI.5989-09.2010. J Neurosci. 2010. PMID: 20427652 Free PMC article.
-
Multiple supporting cell subtypes are capable of spontaneous hair cell regeneration in the neonatal mouse cochlea.Development. 2019 Feb 15;146(4):dev171009. doi: 10.1242/dev.171009. Development. 2019. PMID: 30770379 Free PMC article.
-
Spontaneous Hair Cell Regeneration Is Prevented by Increased Notch Signaling in Supporting Cells.Front Cell Neurosci. 2018 May 4;12:120. doi: 10.3389/fncel.2018.00120. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29780306 Free PMC article.
-
Optogenetics Reveals Roles for Supporting Cells in Force Transmission to and From Outer Hair Cells in the Mouse Cochlea.J Neurosci. 2024 Jan 24;44(4):e1179232023. doi: 10.1523/JNEUROSCI.1179-23.2023. J Neurosci. 2024. PMID: 38050104 Free PMC article.
-
Postnatal development, maturation and aging in the mouse cochlea and their effects on hair cell regeneration.Hear Res. 2013 Mar;297:68-83. doi: 10.1016/j.heares.2012.11.009. Epub 2012 Nov 16. Hear Res. 2013. PMID: 23164734 Free PMC article. Review.
Cited by
-
Perspectives on Human Hearing Loss, Cochlear Regeneration, and the Potential for Hearing Restoration Therapies.Brain Sci. 2020 Oct 20;10(10):756. doi: 10.3390/brainsci10100756. Brain Sci. 2020. PMID: 33092183 Free PMC article. Review.
-
Generation of Atoh1-rtTA transgenic mice: a tool for inducible gene expression in hair cells of the inner ear.Sci Rep. 2014 Nov 3;4:6885. doi: 10.1038/srep06885. Sci Rep. 2014. PMID: 25363458 Free PMC article.
-
Dual expression of Atoh1 and Ikzf2 promotes transformation of adult cochlear supporting cells into outer hair cells.Elife. 2021 Sep 3;10:e66547. doi: 10.7554/eLife.66547. Elife. 2021. PMID: 34477109 Free PMC article.
-
Deterioration of the Medial Olivocochlear Efferent System Accelerates Age-Related Hearing Loss in Pax2-Isl1 Transgenic Mice.Mol Neurobiol. 2016 May;53(4):2368-83. doi: 10.1007/s12035-015-9215-1. Epub 2015 May 20. Mol Neurobiol. 2016. PMID: 25990412
-
Lineage-tracing and translatomic analysis of damage-inducible mitotic cochlear progenitors identifies candidate genes regulating regeneration.PLoS Biol. 2021 Nov 10;19(11):e3001445. doi: 10.1371/journal.pbio.3001445. eCollection 2021 Nov. PLoS Biol. 2021. PMID: 34758021 Free PMC article.
References
-
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. - PubMed
-
- Abrashkin KA, Izumikawa M, Miyazawa T, Wang CH, Crumling MA, Swiderski DL, Beyer LA, Gong TW, Raphael Y. The fate of outer hair cells after acoustic or ototoxic insults. Hear Res. 2006;218:20–29. - PubMed
-
- Ahmad M, Bohne BA, Harding GW. An in vivo tracer study of noise-induced damage to the reticular lamina. Hear Res. 2003;175:82–100. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
