Target-specific IPSC kinetics promote temporal processing in auditory parallel pathways
- PMID: 23345233
- PMCID: PMC3737999
- DOI: 10.1523/JNEUROSCI.2541-12.2013
Target-specific IPSC kinetics promote temporal processing in auditory parallel pathways
Abstract
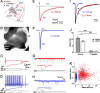
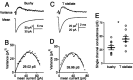
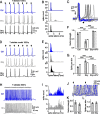
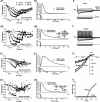
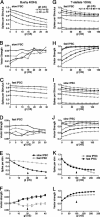
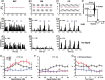
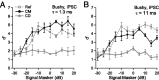
The acoustic environment contains biologically relevant information on timescales from microseconds to tens of seconds. The auditory brainstem nuclei process this temporal information through parallel pathways that originate in the cochlear nucleus from different classes of cells. Although the roles of ion channels and excitatory synapses in temporal processing have been well studied, the contribution of inhibition is less well understood. Here, we show in CBA/CaJ mice that the two major projection neurons of the ventral cochlear nucleus, the bushy and T-stellate cells, receive glycinergic inhibition with different synaptic conductance time courses. Bushy cells, which provide precisely timed spike trains used in sound localization and pitch identification, receive slow inhibitory inputs. In contrast, T-stellate cells, which encode slower envelope information, receive inhibition that is eightfold faster. Both types of inhibition improved the precision of spike timing but engage different cellular mechanisms and operate on different timescales. Computer models reveal that slow IPSCs in bushy cells can improve spike timing on the scale of tens of microseconds. Although fast and slow IPSCs in T-stellate cells improve spike timing on the scale of milliseconds, only fast IPSCs can enhance the detection of narrowband acoustic signals in a complex background. Our results suggest that target-specific IPSC kinetics are critical for the segregated parallel processing of temporal information from the sensory environment.
Figures





References
-
- Altschuler RA, Betz H, Parakkal MH, Reeks KA, Wenthold RJ. Identification of glycinergic synapses in the cochlear nucleus through immunocytochemical localization of the postsynaptic receptor. Brain Res. 1986;369:316–320. - PubMed
-
- Balakrishnan V, Trussell LO. Synaptic inputs to granule cells of the dorsal cochlear nucleus. J Neurophysiol. 2008;99:208–219. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
