Peptide inhibitors disrupt the serotonin 5-HT2C receptor interaction with phosphatase and tensin homolog to allosterically modulate cellular signaling and behavior
- PMID: 23345234
- PMCID: PMC3711763
- DOI: 10.1523/JNEUROSCI.2656-12.2013
Peptide inhibitors disrupt the serotonin 5-HT2C receptor interaction with phosphatase and tensin homolog to allosterically modulate cellular signaling and behavior
Abstract
Serotonin (5-hydroxytryptamine; 5-HT) signaling through the 5-HT(2C) receptor (5-HT(2C)R) is essential in normal physiology, whereas aberrant 5-HT(2C)R function is thought to contribute to the pathogenesis of multiple neural disorders. The 5-HT(2C)R interacts with specific protein partners, but the impact of such interactions on 5-HT(2C)R function is poorly understood. Here, we report convergent cellular and behavioral data that the interaction between the 5-HT(2C)R and protein phosphatase and tensin homolog (PTEN) serves as a regulatory mechanism to control 5-HT(2C)R-mediated biology but not that of the closely homologous 5-HT(2A)R. A peptide derived from the third intracellular loop of the human 5-HT(2C)R [3L4F (third loop, fourth fragment)] disrupted the association, allosterically augmented 5-HT(2C)R-mediated signaling in live cells, and acted as a positive allosteric modulator in rats in vivo. We identified the critical residues within an 8 aa fragment of the 3L4F peptide that maintained efficacy (within the picomolar range) in live cells similar to that of the 3L4F peptide. Last, molecular modeling identified key structural features and potential interaction sites of the active 3L4F peptides against PTEN. These compelling data demonstrate the specificity and importance of this protein assembly in cellular events and behaviors mediated by 5-HT(2C)R signaling and provide a chemical guidepost to the future development of drug-like peptide or small-molecule inhibitors as neuroprobes to study 5-HT(2C)R allostery and therapeutics for 5-HT(2C)R-mediated disorders.
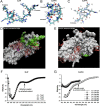
Figures




References
-
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. - PubMed
-
- Agnati LF, Guidolin D, Leo G, Guescini M, Pizzi M, Stocchi V, Spano PF, Ghidoni R, Ciruela F, Genedani S, Fuxe K. Possible new targets for GPCR modulation: allosteric interactions, plasma membrane domains, intercellular transfer and epigenetic mechanisms. J Recept Signal Transduct Res. 2011;31:315–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
