Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy
- PMID: 23345237
- PMCID: PMC3711605
- DOI: 10.1523/JNEUROSCI.3191-12.2013
Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy
Abstract
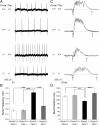
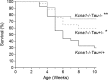
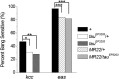
Neuronal network hyperexcitability underlies the pathogenesis of seizures and is a component of some degenerative neurological disorders such as Alzheimer's disease (AD). Recently, the microtubule-binding protein tau has been implicated in the regulation of network synchronization. Genetic removal of Mapt, the gene encoding tau, in AD models overexpressing amyloid-β (Aβ) decreases hyperexcitability and normalizes the excitation/inhibition imbalance. Whether this effect of tau removal is specific to Aβ mouse models remains to be determined. Here, we examined tau as an excitability modifier in the non-AD nervous system using genetic deletion of tau in mouse and Drosophila models of hyperexcitability. Kcna1(-/-) mice lack Kv1.1-delayed rectifier currents and exhibit severe spontaneous seizures, early lethality, and megencephaly. Young Kcna1(-/-) mice retained wild-type levels of Aβ, tau, and tau phospho-Thr(231). Decreasing tau in Kcna1(-/-) mice reduced hyperexcitability and alleviated seizure-related comorbidities. Tau reduction decreased Kcna1(-/-) video-EEG recorded seizure frequency and duration as well as normalized Kcna1(-/-) hippocampal network hyperexcitability in vitro. Additionally, tau reduction increased Kcna1(-/-) survival and prevented megencephaly and hippocampal hypertrophy, as determined by MRI. Bang-sensitive Drosophila mutants display paralysis and seizures in response to mechanical stimulation, providing a complementary excitability assay for epistatic interactions. We found that tau reduction significantly decreased seizure sensitivity in two independent bang-sensitive mutant models, kcc and eas. Our results indicate that tau plays a general role in regulating intrinsic neuronal network hyperexcitability independently of Aβ overexpression and suggest that reducing tau function could be a viable target for therapeutic intervention in seizure disorders and antiepileptogenesis.
Figures



References
-
- Adelman JP, Bond CT, Pessia M, Maylie J. Episodic ataxia results from voltage-dependent potassium channels with altered functions. Neuron. 1995;15:1449–1454. - PubMed
-
- Almgren M, Persson AS, Fenghua C, Witgen BM, Schalling M, Nyengaard JR, Lavebratt C. Lack of potassium channel induces proliferation and survival causing increased neurogenesis and twofold hippocampus enlargement. Hippocampus. 2007;17:292–304. - PubMed
-
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 2002;103:26–35. - PubMed
-
- Buerger K, Teipel SJ, Zinkowski R, Blennow K, Arai H, Engel R, Hofmann-Kiefer K, McCulloch C, Ptok U, Heun R, Andreasen N, DeBernardis J, Kerkman D, Moeller H, Davies P, Hampel H. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology. 2002;59:627–629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
