Calcium channel agonists protect against neuromuscular dysfunction in a genetic model of TDP-43 mutation in ALS
- PMID: 23345247
- PMCID: PMC6618732
- DOI: 10.1523/JNEUROSCI.4003-12.2013
Calcium channel agonists protect against neuromuscular dysfunction in a genetic model of TDP-43 mutation in ALS
Abstract
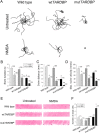
TAR DNA binding protein (TDP-43, encoded by the TARDBP gene) has recently been shown to be associated with amyotrophic lateral sclerosis (ALS), but the early pathophysiological deficits causing impairment in motor function are unknown. Here we expressed the wild-type human gene (wtTARDBP) or the ALS mutation G348C (mutTARDBP) in zebrafish larvae and characterized their motor (swimming) activity and the structure and function of their neuromuscular junctions (NMJs). Of these groups only mutTARDBP larvae showed impaired swimming and increased motoneuron vulnerability with reduced synaptic fidelity, reduced quantal transmission, and more orphaned presynaptic and postsynaptic structures at the NMJ. Remarkably, all behavioral and cellular features were stabilized by chronic treatment with either of the L-type calcium channel agonists FPL 64176 or Bay K 8644. These results indicate that expression of mutTARDBP results in defective NMJs and that calcium channel agonists could be novel therapeutics for ALS.
Figures





References
-
- Arenson MS, Evans SC. Activation of protein kinase C increases acetylcholine release from frog motor nerves by a direct action on L-type Ca2+ channels and apparently not by depolarisation of the terminal. Neuroscience. 2001;104:1157–1164. - PubMed
-
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994;330:585–591. - PubMed
-
- Bories C, Amendola J, Lamotte d'Incamps B, Durand J. Early electrophysiological abnormalities in lumbar motoneurons in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2007;25:451–459. - PubMed
-
- Buss RR, Drapeau P. Synaptic drive to motoneurons during fictive swimming in the developing zebrafish. J Neurophysiol. 2001;86:197–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
