Development of a panel of recombinase polymerase amplification assays for detection of biothreat agents
- PMID: 23345286
- PMCID: PMC3666764
- DOI: 10.1128/JCM.02704-12
Development of a panel of recombinase polymerase amplification assays for detection of biothreat agents
Abstract
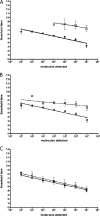
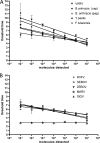
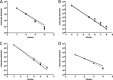
Syndromic panels for infectious disease have been suggested to be of value in point-of-care diagnostics for developing countries and for biodefense. To test the performance of isothermal recombinase polymerase amplification (RPA) assays, we developed a panel of 10 RPAs for biothreat agents. The panel included RPAs for Francisella tularensis, Yersinia pestis, Bacillus anthracis, variola virus, and reverse transcriptase RPA (RT-RPA) assays for Rift Valley fever virus, Ebola virus, Sudan virus, and Marburg virus. Their analytical sensitivities ranged from 16 to 21 molecules detected (probit analysis) for the majority of RPA and RT-RPA assays. A magnetic bead-based total nucleic acid extraction method was combined with the RPAs and tested using inactivated whole organisms spiked into plasma. The RPA showed comparable sensitivities to real-time RCR assays in these extracts. The run times of the assays at 42°C ranged from 6 to 10 min, and they showed no cross-detection of any of the target genomes of the panel nor of the human genome. The RPAs therefore seem suitable for the implementation of syndromic panels onto microfluidic platforms.
Figures

References
-
- Peeling RW, Mabey D. 2010. Point-of-care tests for diagnosing infections in the developing world. Clin. Microbiol. Infect. 16:1062–1069 - PubMed
-
- Cuchacovich R. 2006. Clinical implications of the polymerase chain reaction: an update. Dis. Clin. N. Am. 20:735–758 - PubMed
-
- Carman B. 2001. Molecular techniques should now replace cell culture in diagnostic virology laboratories. Rev. Med. Virol. 11:347–349 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

