Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis
- PMID: 23345334
- PMCID: PMC3578001
- DOI: 10.4049/jimmunol.1202535
Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis
Abstract
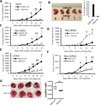
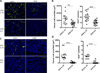
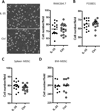
IL-35 is a member of the IL-12 family of cytokines that is comprised of an IL-12 p35 subunit and an IL-12 p40-related protein subunit, EBV-induced gene 3 (EBI3). IL-35 functions through IL-35R and has a potent immune-suppressive activity. Although IL-35 was demonstrated to be produced by regulatory T cells, gene-expression analysis revealed that it is likely to have a wider distribution, including expression in cancer cells. In this study, we demonstrated that IL-35 is produced in human cancer tissues, such as large B cell lymphoma, nasopharyngeal carcinoma, and melanoma. To determine the roles of tumor-derived IL-35 in tumorigenesis and tumor immunity, we generated IL-35-producing plasmacytoma J558 and B16 melanoma cells and observed that the expression of IL-35 in cancer cells does not affect their growth and survival in vitro, but it stimulates tumorigenesis in both immune-competent and Rag1/2-deficient mice. Tumor-derived IL-35 increases CD11b(+)Gr1(+) myeloid cell accumulation in the tumor microenvironment and, thereby, promotes tumor angiogenesis. In immune-competent mice, spontaneous CTL responses to tumors are diminished. IL-35 does not directly inhibit tumor Ag-specific CD8(+) T cell activation, differentiation, and effector functions. However, IL-35-treated cancer cells had increased expression of gp130 and reduced sensitivity to CTL destruction. Thus, our study indicates novel functions for IL-35 in promoting tumor growth via the enhancement of myeloid cell accumulation, tumor angiogenesis, and suppression of tumor immunity.
Figures






References
-
- Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. - PubMed
-
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. - PubMed
-
- Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L'Hermine A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol. 2008;181:6898–6905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

