Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury
- PMID: 23345421
- PMCID: PMC3568296
- DOI: 10.1073/pnas.1214136110
Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury
Abstract
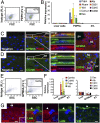
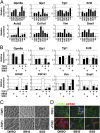
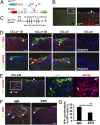
In many organs, myofibroblasts play a major role in the scarring process in response to injury. In liver fibrogenesis, hepatic stellate cells (HSCs) are thought to transdifferentiate into myofibroblasts, but the origins of both HSCs and myofibroblasts remain elusive. In the developing liver, lung, and intestine, mesothelial cells (MCs) differentiate into specific mesenchymal cell types; however, the contribution of this differentiation to organ injury is unknown. In the present study, using mouse models, conditional cell lineage analysis has demonstrated that MCs expressing Wilms tumor 1 give rise to HSCs and myofibroblasts during liver fibrogenesis. Primary MCs, isolated from adult mouse liver using antibodies against glycoprotein M6a, undergo myofibroblastic transdifferentiation. Antagonism of TGF-β signaling suppresses transition of MCs to mesenchymal cells both in vitro and in vivo. These results indicate that MCs undergo mesothelial-mesenchymal transition and participate in liver injury via differentiation to HSCs and myofibroblasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mutsaers SE. The mesothelial cell. Int J Biochem Cell Biol. 2004;36(1):9–16. - PubMed
-
- Yung S, Li FK, Chan TM. Peritoneal mesothelial cell culture and biology. Perit Dial Int. 2006;26(2):162–173. - PubMed
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. - PubMed
-
- Patel P, et al. Smad3-dependent and -independent pathways are involved in peritoneal membrane injury. Kidney Int. 2010;77(4):319–328. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

