Contribution of Thy1+ NK cells to protective IFN-γ production during Salmonella typhimurium infections
- PMID: 23345426
- PMCID: PMC3568339
- DOI: 10.1073/pnas.1222047110
Contribution of Thy1+ NK cells to protective IFN-γ production during Salmonella typhimurium infections
Abstract
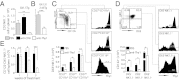
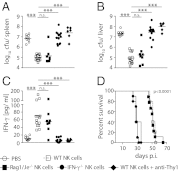
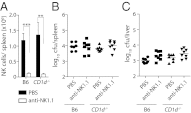
IFN-γ is critical for immunity against infections with intracellular pathogens, such as Salmonella enterica. However, which of the many cell types capable of producing IFN-γ controls Salmonella infections remains unclear. Using a mouse model of systemic Salmonella infection, we observed that only a lack of all lymphocytes or CD90 (Thy1)(+) cells, but not the absence of T cells, Retinoic acid-related orphan receptor (ROR)-γt-dependent lymphocytes, (NK)1.1(+) cells, natural killer T (NKT), and/or B cells alone, replicated the highly susceptible phenotype of IFN-γ-deficient mice to Salmonella infection. A combination of antibody depletions and adoptive transfer experiments revealed that early protective IFN-γ was provided by Thy1-expressing natural killer (NK) cells and that these cells improved antibacterial immunity through the provision of IFN-γ. Further analysis of NK cells producing IFN-γ in response to Salmonella indicated that less mature NK cells were more efficient at mediating antibacterial effector function than terminally differentiated NK cells. Inspired by recent reports of Thy1(+) NK cells contributing to immune memory, we analyzed their role in secondary protection against otherwise lethal WT Salmonella infections. Notably, we observed that a newly generated Salmonella vaccine strain not only conferred superior protection compared with conventional regimens but that this enhanced efficiency of recall immunity was afforded by incorporating CD4(-)CD8(-)Thy1(+) cells into the secondary response. Taken together, these findings demonstrate that Thy1-expressing NK cells play an important role in antibacterial immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6(1):53–66. - PubMed
-
- VanCott JL, et al. Regulation of host immune responses by modification of Salmonella virulence genes. Nat Med. 1998;4(11):1247–1252. - PubMed
-
- de Jong R, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280(5368):1435–1438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

