UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes
- PMID: 23345429
- PMCID: PMC3568351
- DOI: 10.1073/pnas.1215555110
UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes
Abstract
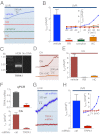
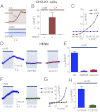
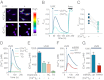
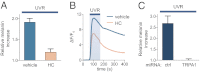
Human skin is constantly exposed to solar ultraviolet radiation (UVR), the most prevalent environmental carcinogen. Humans have the unique ability among mammals to respond to UVR by increasing their skin pigmentation, a protective process driven by melanin synthesis in epidermal melanocytes. The molecular mechanisms used by melanocytes to detect and respond to long-wavelength UVR (UVA) are not well understood. We recently identified a UVA phototransduction pathway in melanocytes that is mediated by G protein-coupled receptors and leads to rapid calcium mobilization. Here we report that in human epidermal melanocytes physiological doses of UVR activate a retinal-dependent current mediated by transient receptor potential A1 (TRPA1) ion channels. The TRPA1 photocurrent is UVA-specific and requires G protein and phospholipase C signaling, thus contributing to UVA-induced calcium responses to mediate downstream cellular effects and providing evidence for TRPA1 function in mammalian phototransduction. Remarkably, TRPA1 activation is required for the UVR-induced and retinal-dependent early increase in cellular melanin. Our results show that TRPA1 is essential for a unique extraocular phototransduction pathway in human melanocytes that is activated by physiological doses of UVR and results in early melanin synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445(7130):843–850. - PubMed
-
- Kobayashi N, et al. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J Invest Dermatol. 1998;110(5):806–810. - PubMed
-
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224(5216):285–287. - PubMed
-
- Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230(4729):1040–1043. - PubMed
-
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8(4):643–651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

