Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells
- PMID: 23345430
- PMCID: PMC3568356
- DOI: 10.1073/pnas.1219002110
Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells
Abstract
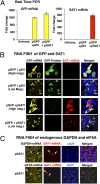
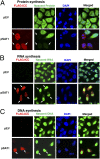
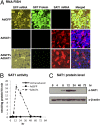
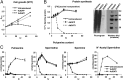
The polyamines, putrescine, spermidine, and spermine, are essential polycations, intimately involved in the regulation of cellular proliferation. Although polyamines exert dynamic effects on the conformation of nucleic acids and macromolecular synthesis in vitro, their specific functions in vivo are poorly understood. We investigated the cellular function of polyamines by overexpression of a key catabolic enzyme, spermidine/spermine N(1)-acetyltransferase 1 (SAT1) in mammalian cells. Transient cotransfection of HeLa cells with GFP and SAT1 vectors suppressed GFP protein expression without lowering its mRNA level, an indication that the block in GFP expression was not at transcription, but at translation. Fluorescence single-cell imaging also revealed specific inhibition of endogenous protein synthesis in the SAT1 overexpressing cells, without any inhibition of synthesis of DNA or RNA. Overexpression of SAT1 using a SAT1 adenovirus led to rapid depletion of cellular spermidine and spermine, total inhibition of protein synthesis, and growth arrest within 24 h. The SAT1 effect is most likely due to depletion of spermidine and spermine, because stable polyamine analogs that are not substrates for SAT1 restored GFP and endogenous protein synthesis. Loss of polysomes with increased 80S monosomes in the polyamine-depleted cells suggests a direct role for polyamines in translation initiation. Our data provide strong evidence for a primary function of polyamines, spermidine and spermine, in translation in mammalian cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. - PubMed
-
- Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42(1):39–51. - PubMed
-
- Gerner EW, Meyskens FL., Jr Polyamines and cancer: Old molecules, new understanding. Nat Rev Cancer. 2004;4(10):781–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

