Susceptibility of human iris stromal cells to herpes simplex virus 1 entry
- PMID: 23345512
- PMCID: PMC3624200
- DOI: 10.1128/JVI.03235-12
Susceptibility of human iris stromal cells to herpes simplex virus 1 entry
Abstract
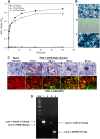
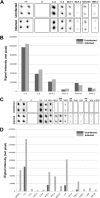
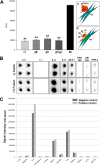
Ocular herpes simplex virus 1 (HSV-1) infection can lead to multiple complications, including iritis, an inflammation of the iris. Here, we use human iris stroma cells as a novel in vitro model to demonstrate HSV-1 entry and the inflammatory mediators that can damage the iris. The upregulated cytokines observed in this study provide a new understanding of the intrinsic immune mechanisms that can contribute to the onset of iritis.
Figures
Similar articles
-
A synthetic glycosaminoglycan mimetic blocks HSV-1 infection in human iris stromal cells.Antiviral Res. 2019 Jan;161:154-162. doi: 10.1016/j.antiviral.2018.11.007. Epub 2018 Nov 24. Antiviral Res. 2019. PMID: 30481525
-
Herpes simplex virus type-1 (HSV-1) entry into human mesenchymal stem cells is heavily dependent on heparan sulfate.J Biomed Biotechnol. 2011;2011:264350. doi: 10.1155/2011/264350. Epub 2011 Jun 21. J Biomed Biotechnol. 2011. PMID: 21799659 Free PMC article.
-
Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1.Nature. 2010 Oct 14;467(7317):859-62. doi: 10.1038/nature09420. Nature. 2010. PMID: 20944748
-
Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo.J Biol Chem. 2011 Jul 15;286(28):25406-15. doi: 10.1074/jbc.M110.201103. Epub 2011 May 19. J Biol Chem. 2011. PMID: 21596749 Free PMC article.
-
Herpesvirus entry mediator on radiation-resistant cell lineages promotes ocular herpes simplex virus 1 pathogenesis in an entry-independent manner.mBio. 2015 Oct 20;6(5):e01532-15. doi: 10.1128/mBio.01532-15. mBio. 2015. PMID: 26489863 Free PMC article.
Cited by
-
HSV-1 interaction to 3-O-sulfated heparan sulfate in mouse-derived DRG explant and profiles of inflammatory markers during virus infection.J Neurovirol. 2017 Jun;23(3):483-491. doi: 10.1007/s13365-017-0521-4. Epub 2017 Mar 21. J Neurovirol. 2017. PMID: 28326469 Free PMC article.
-
Iridian anterior segment OCT in rubella uveitis syndrome and cytomegalovirus anterior uveitis: a comparative study.Graefes Arch Clin Exp Ophthalmol. 2022 Nov;260(11):3647-3655. doi: 10.1007/s00417-022-05733-3. Epub 2022 Jun 16. Graefes Arch Clin Exp Ophthalmol. 2022. PMID: 35708847
-
A role for 3-O-sulfated heparan sulfate in promoting human cytomegalovirus infection in human iris cells.J Virol. 2015 May;89(9):5185-92. doi: 10.1128/JVI.00109-15. Epub 2015 Feb 25. J Virol. 2015. PMID: 25717110 Free PMC article.
-
Induction of Filopodia During Cytomegalovirus Entry Into Human Iris Stromal Cells.Front Microbiol. 2022 Apr 5;13:834927. doi: 10.3389/fmicb.2022.834927. eCollection 2022. Front Microbiol. 2022. PMID: 35450284 Free PMC article.
-
High Preventive Effect of G2-S16 Anionic Carbosilane Dendrimer against Sexually Transmitted HSV-2 Infection.Molecules. 2020 Jun 28;25(13):2965. doi: 10.3390/molecules25132965. Molecules. 2020. PMID: 32605185 Free PMC article. Review.
References
-
- Dawson CR, Togni B. 1976. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Surv. Ophthalmol. 21:121–135 - PubMed
-
- Rathinam SR, Namperumalsamy P. 2007. Global variation and pattern changes in epidemiology of uveitis. Indian J. Ophthalmol. 55:173–183 - PubMed
-
- Teitelbaum CS, Streeten BW, Dawson CR. 1987. Histopathology of herpes simplex virus keratouveitis. Curr. Eye Res. 6:189–194 - PubMed
-
- Sungur GK, Hazirolan D, Yalvac IS, Ozer PA, Aslan BS, Duman S. 2010. Incidence and prognosis of ocular hypertension secondary to viral uveitis. Int. Ophthalmol. 30:191–194 - PubMed
-
- Tugal-Tutkun I, Ötük-Yasar B, Altinkurt E. 2010. Clinical features and prognosis of herpetic anterior uveitis: a retrospective study of 111 cases. Int. Ophthalmol. 30:559–565 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

