Identification of a role for the trans-Golgi network in human papillomavirus 16 pseudovirus infection
- PMID: 23345514
- PMCID: PMC3624235
- DOI: 10.1128/JVI.03222-12
Identification of a role for the trans-Golgi network in human papillomavirus 16 pseudovirus infection
Abstract
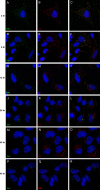
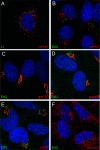
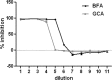
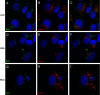
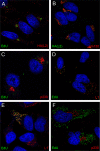
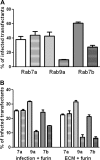
Human papillomavirus 16 (HPV16) enters its host cells by a process that most closely resembles macropinocytosis. Uncoating occurs during passage through the endosomal compartment, and the low pH encountered in this environment is essential for infection. Furin cleavage of the minor capsid protein, L2, and cyclophilin B-mediated separation of L2 and the viral genome from the major capsid protein, L1, are necessary for escape from the late endosome (LE). Following this exodus, L2 and the genome are found colocalized at the ND10 nuclear subdomain, which is essential for efficient pseudogenome expression. However, the route by which L2 and the genome traverse the intervening cytoplasm between these two subcellular compartments has not been determined. This study extends our understanding of this phase in PV entry in demonstrating the involvement of the Golgi complex. With confocal microscopic analyses involving 5-ethynyl-2'-deoxyuridine (EdU)-labeled pseudogenomes and antibodies to virion and cellular proteins, we found that the viral pseudogenome and L2 travel to the trans-Golgi network (TGN) following exit from the LE, while L1 is retained. This transit is dependent upon furin cleavage of L2 and can be prevented pharmacologically with either brefeldin A or golgicide A, inhibitors of anterograde and retrograde Golgi trafficking. Additionally, Rab9a and Rab7b were determined to be mediators of this transit, as expression of dominant negative versions of these proteins, but not Rab7a, significantly inhibited HPV16 pseudovirus infection.
Figures
Similar articles
-
The nuclear retention signal of HPV16 L2 protein is essential for incoming viral genome to transverse the trans-Golgi network.Virology. 2014 Jun;458-459:93-105. doi: 10.1016/j.virol.2014.04.024. Epub 2014 May 8. Virology. 2014. PMID: 24928042 Free PMC article.
-
HPV16 entry requires dynein for minus-end transport and utilizes kinesin Kif11 for plus-end transport along microtubules during mitosis.J Virol. 2025 Jan 31;99(1):e0093724. doi: 10.1128/jvi.00937-24. Epub 2024 Dec 4. J Virol. 2025. PMID: 39629998 Free PMC article.
-
Rab6a enables BICD2/dynein-mediated trafficking of human papillomavirus from the trans-Golgi network during virus entry.mBio. 2024 Nov 13;15(11):e0281124. doi: 10.1128/mbio.02811-24. Epub 2024 Oct 21. mBio. 2024. PMID: 39431827 Free PMC article.
-
L2, the minor capsid protein of papillomavirus.Virology. 2013 Oct;445(1-2):175-86. doi: 10.1016/j.virol.2013.04.017. Epub 2013 May 17. Virology. 2013. PMID: 23689062 Free PMC article. Review.
-
HPV16 Entry into Epithelial Cells: Running a Gauntlet.Viruses. 2021 Dec 8;13(12):2460. doi: 10.3390/v13122460. Viruses. 2021. PMID: 34960729 Free PMC article. Review.
Cited by
-
Incoming human papillomavirus type 16 genome resides in a vesicular compartment throughout mitosis.Proc Natl Acad Sci U S A. 2016 May 31;113(22):6289-94. doi: 10.1073/pnas.1600638113. Epub 2016 May 17. Proc Natl Acad Sci U S A. 2016. PMID: 27190090 Free PMC article.
-
Identification of TRAPPC8 as a host factor required for human papillomavirus cell entry.PLoS One. 2013 Nov 14;8(11):e80297. doi: 10.1371/journal.pone.0080297. eCollection 2013. PLoS One. 2013. PMID: 24244674 Free PMC article.
-
Opportunities and challenges for human papillomavirus vaccination in cancer.Nat Rev Cancer. 2018 Apr;18(4):240-254. doi: 10.1038/nrc.2018.13. Epub 2018 Mar 2. Nat Rev Cancer. 2018. PMID: 29497146 Free PMC article. Review.
-
Advances in the Prevention of Cervical Cancer by Anti-Human Papillomavirus Agents.Cancer Med. 2025 Apr;14(7):e70847. doi: 10.1002/cam4.70847. Cancer Med. 2025. PMID: 40189844 Free PMC article. Review.
-
The CD63-Syntenin-1 Complex Controls Post-Endocytic Trafficking of Oncogenic Human Papillomaviruses.Sci Rep. 2016 Aug 31;6:32337. doi: 10.1038/srep32337. Sci Rep. 2016. PMID: 27578500 Free PMC article.
References
-
- Liu WJ, Gissmann L, Sun XY, Kanjanahaluethai A, Muller M, Doorbar J, Zhou J. 1997. Sequence close to the N-terminus of L2 protein is displayed on the surface of bovine papillomavirus type 1 virions. Virology 227:474–483 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

