Glycoproteomic analysis of the secretome of human endothelial cells
- PMID: 23345538
- PMCID: PMC3617342
- DOI: 10.1074/mcp.M112.024018
Glycoproteomic analysis of the secretome of human endothelial cells
Abstract
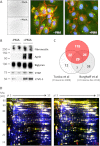
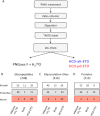
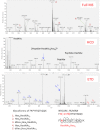
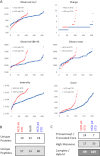
Previous proteomics studies have partially unraveled the complexity of endothelial protein secretion but have not investigated glycosylation, a key modification of secreted and membrane proteins for cell communication. In this study, human umbilical vein endothelial cells were kept in serum-free medium before activation by phorbol-12-myristate-13 acetate, a commonly used secretagogue that induces exocytosis of endothelial vesicles. In addition to 123 secreted proteins, the secretome was particularly rich in membrane proteins. Glycopeptides were enriched by zwitterionic hydrophilic interaction liquid chromatography resins and were either treated with PNGase F and H2(18)O or directly analyzed using a recently developed workflow combining higher-energy C-trap dissociation (HCD) with electron-transfer dissociation (ETD) for a hybrid linear ion trap-orbitrap mass spectrometer. After deglycosylation with PNGase F in the presence of H2(18)O, 123 unique peptides displayed (18)O-deamidation of asparagine, corresponding to 86 proteins with a total of 121 glycosylation sites. Direct glycopeptide analysis via HCD-ETD identified 131 glycopeptides from 59 proteins and 118 glycosylation sites, of which 41 were known, 51 were predicted, and 26 were novel. Two methods were compared: alternating HCD-ETD and HCD-product-dependent ETD. The former detected predominantly high-intensity, multiply charged glycopeptides, whereas the latter preferentially selected precursors with complex/hybrid glycans for fragmentation. Validation was performed by means of glycoprotein enrichment and analysis of the input, the flow-through, and the bound fraction. This study represents the most comprehensive characterization of endothelial protein secretion to date and demonstrates the potential of new HCD-ETD workflows for determining the glycosylation status of complex biological samples.
Figures

Similar articles
-
Higher energy collision dissociation (HCD) product ion-triggered electron transfer dissociation (ETD) mass spectrometry for the analysis of N-linked glycoproteins.J Proteome Res. 2012 Sep 7;11(9):4517-25. doi: 10.1021/pr300257c. Epub 2012 Aug 6. J Proteome Res. 2012. PMID: 22800195
-
Enhanced mass spectrometric mapping of the human GalNAc-type O-glycoproteome with SimpleCells.Mol Cell Proteomics. 2013 Apr;12(4):932-44. doi: 10.1074/mcp.O112.021972. Epub 2013 Feb 11. Mol Cell Proteomics. 2013. PMID: 23399548 Free PMC article.
-
Simultaneous glycan-peptide characterization using hydrophilic interaction chromatography and parallel fragmentation by CID, higher energy collisional dissociation, and electron transfer dissociation MS applied to the N-linked glycoproteome of Campylobacter jejuni.Mol Cell Proteomics. 2011 Feb;10(2):M000031-MCP201. doi: 10.1074/mcp.M000031-MCP201. Epub 2010 Apr 1. Mol Cell Proteomics. 2011. PMID: 20360033 Free PMC article.
-
[Recent advances in glycopeptide enrichment and mass spectrometry data interpretation approaches for glycoproteomics analyses].Se Pu. 2021 Oct;39(10):1045-1054. doi: 10.3724/SP.J.1123.2021.06011. Se Pu. 2021. PMID: 34505426 Free PMC article. Review. Chinese.
-
Glycoproteomic analysis of antibodies.Mol Cell Proteomics. 2013 Apr;12(4):856-65. doi: 10.1074/mcp.R112.026005. Epub 2013 Jan 16. Mol Cell Proteomics. 2013. PMID: 23325769 Free PMC article. Review.
Cited by
-
Adapting extracellular matrix proteomics for clinical studies on cardiac remodeling post-myocardial infarction.Clin Proteomics. 2016 Sep 15;13:19. doi: 10.1186/s12014-016-9120-2. eCollection 2016. Clin Proteomics. 2016. PMID: 27651752 Free PMC article. Review.
-
Large-scale intact glycopeptide identification by Mascot database search.Sci Rep. 2018 Feb 1;8(1):2117. doi: 10.1038/s41598-018-20331-2. Sci Rep. 2018. PMID: 29391424 Free PMC article.
-
Liquid chromatography-tandem mass spectrometry-based fragmentation analysis of glycopeptides.Glycoconj J. 2016 Jun;33(3):261-72. doi: 10.1007/s10719-016-9649-3. Epub 2016 Jan 18. Glycoconj J. 2016. PMID: 26780731 Review.
-
Comparative N-Glycoproteomics Analysis of Clinical Samples Via Different Mass Spectrometry Dissociation Methods.Front Chem. 2022 Feb 24;10:839470. doi: 10.3389/fchem.2022.839470. eCollection 2022. Front Chem. 2022. PMID: 35281567 Free PMC article.
-
Quiescin Sulfhydryl Oxidase 1 (QSOX1) Secreted by Lung Cancer Cells Promotes Cancer Metastasis.Int J Mol Sci. 2018 Oct 17;19(10):3213. doi: 10.3390/ijms19103213. Int J Mol Sci. 2018. PMID: 30336636 Free PMC article.
References
-
- Mayr M., Mayr U., Chung Y. L., Yin X., Griffiths J. R., Xu Q. (2004) Vascular proteomics: linking proteomic and metabolomic changes. Proteomics 4, 3751–3761 - PubMed
-
- Pula G., Perera S., Prokopi M., Sidibe A., Boulanger C. M., Mayr M. (2008) Proteomic analysis of secretory proteins and vesicles in vascular research. Proteomics Clin. Appl. 2, 882–891 - PubMed
-
- Pober J. S., Sessa W. C. (2007) Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7, 803–815 - PubMed
-
- Cai H., Harrison D. G. (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 87, 840–844 - PubMed
-
- Libby P., Simon D. I. (2001) Inflammation and thrombosis: the clot thickens. Circulation 103, 1718–1720 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

