The role of MET receptor tyrosine kinase in non-small cell lung cancer and clinical development of targeted anti-MET agents
- PMID: 23345546
- PMCID: PMC3579594
- DOI: 10.1634/theoncologist.2012-0262
The role of MET receptor tyrosine kinase in non-small cell lung cancer and clinical development of targeted anti-MET agents
Abstract
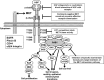
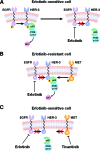
A better understanding of the pathophysiology and evolution of non-small cell lung cancer (NSCLC) has identified a number of molecular targets and spurred development of novel targeted therapeutic agents. The MET receptor tyrosine kinase and its ligand hepatocyte growth factor (HGF) are implicated in tumor cell proliferation, migration, invasion, and angiogenesis in a broad spectrum of human cancers, including NSCLC. Amplification of MET has been reported in approximately 5%-22% of lung tumors with acquired resistance to small-molecule inhibitors of the epidermal growth factor receptor (EGFR). Resistance to EGFR inhibitors is likely mediated through downstream activation of the phosphoinositide 3-kinase /AKT pathway. Simultaneous treatment of resistant tumors with a MET inhibitor plus an EGFR inhibitor can abrogate activation of downstream effectors of cell growth, proliferation, and survival, thereby overcoming acquired resistance to EGFR inhibitors. Development and preclinical testing of multiple agents targeting the HGF-MET pathway, including monoclonal antibodies targeting HGF or the MET receptor and small-molecule inhibitors of the MET tyrosine kinase, have confirmed the crucial role of this pathway in NSCLC. Several agents are now in phase III clinical development for the treatment of NSCLC. This review summarizes the role of MET in the pathophysiology of NSCLC and in acquired resistance to EGFR inhibitors and provides an update on progress in the clinical development of inhibitors of MET for treatment of NSCLC.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

References
-
- Surveillance, Epidemiology, and End Results. Table 1.1. Estimated New Cancer Cases and Deaths for 2010. All Races, By Sex. [accessed September 4, 2012]. Available at http://seer.cancer.gov/csr/1975_2007/results_single/sect_01_table.01.pdf.
-
- Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65:1479–1488. - PubMed
-
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. - PubMed
-
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. - PubMed
-
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

