Major histocompatibility complex peptide ligands as olfactory cues in human body odour assessment
- PMID: 23345577
- PMCID: PMC3574394
- DOI: 10.1098/rspb.2012.2889
Major histocompatibility complex peptide ligands as olfactory cues in human body odour assessment
Erratum in
- Proc Biol Sci. 2013 Mar 22;280(1755):20130381
Abstract
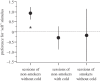
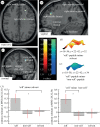
In many animal species, social communication and mate choice are influenced by cues encoded by the major histocompatibility complex (MHC). The mechanism by which the MHC influences sexual selection is a matter of intense debate. In mice, peptide ligands of MHC molecules activate subsets of vomeronasal and olfactory sensory neurons and influence social memory formation; in sticklebacks, such peptides predictably modify the outcome of mate choice. Here, we examine whether this evolutionarily conserved mechanism of interindividual communication extends to humans. In psychometric tests, volunteers recognized the supplementation of their body odour by MHC peptides and preferred 'self' to 'non-self' ligands when asked to decide whether the modified odour smelled 'like themselves' or 'like their favourite perfume'. Functional magnetic resonance imaging indicated that 'self'-peptides specifically activated a region in the right middle frontal cortex. Our results suggest that despite the absence of a vomeronasal organ, humans have the ability to detect and evaluate MHC peptides in body odour. This may provide a basis for the sensory evaluation of potential partners during human mate choice.
Figures
Comment in
-
A human chemosensory modality to detect peptides in the nose?Proc Biol Sci. 2013 Dec 11;281(1776):20131678. doi: 10.1098/rspb.2013.1678. Print 2014 Feb 7. Proc Biol Sci. 2013. PMID: 24335978 Free PMC article. No abstract available.
Similar articles
-
Odour-based discrimination of similarity at the major histocompatibility complex in birds.Proc Biol Sci. 2017 Jan 11;284(1846):20162466. doi: 10.1098/rspb.2016.2466. Proc Biol Sci. 2017. PMID: 28077776 Free PMC article.
-
Mate choice decisions of stickleback females predictably modified by MHC peptide ligands.Proc Natl Acad Sci U S A. 2005 Mar 22;102(12):4414-8. doi: 10.1073/pnas.0408264102. Epub 2005 Mar 8. Proc Natl Acad Sci U S A. 2005. PMID: 15755811 Free PMC article.
-
Major histocompatibility complex-associated odour preferences and human mate choice: near and far horizons.Philos Trans R Soc Lond B Biol Sci. 2020 Jun 8;375(1800):20190260. doi: 10.1098/rstb.2019.0260. Epub 2020 Apr 20. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32306884 Free PMC article.
-
MHC peptides and the sensory evaluation of genotype.Trends Neurosci. 2006 Feb;29(2):100-7. doi: 10.1016/j.tins.2005.11.006. Epub 2005 Dec 6. Trends Neurosci. 2006. PMID: 16337283 Review.
-
Olfactory cues associated with the major histocompatibility complex.Genetica. 1998-1999;104(3):191-7. doi: 10.1023/a:1026402531196. Genetica. 1998. PMID: 10386382 Review.
Cited by
-
Reply to A human chemo-sensory modality to detect peptides in the nose? by A. Natsch.Proc Biol Sci. 2013 Dec 11;281(1776):20132816. doi: 10.1098/rspb.2013.2816. Print 2014 Feb 7. Proc Biol Sci. 2013. PMID: 24335988 Free PMC article. No abstract available.
-
The specific biochemistry of human axilla odour formation viewed in an evolutionary context.Philos Trans R Soc Lond B Biol Sci. 2020 Jun 8;375(1800):20190269. doi: 10.1098/rstb.2019.0269. Epub 2020 Apr 20. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32306870 Free PMC article. Review.
-
Major histocompatibility complex-linked social signalling affects female fertility.Proc Biol Sci. 2017 Dec 6;284(1868):20171824. doi: 10.1098/rspb.2017.1824. Proc Biol Sci. 2017. PMID: 29212724 Free PMC article.
-
Smelling phenomenal.Front Psychol. 2014 Jul 10;5:713. doi: 10.3389/fpsyg.2014.00713. eCollection 2014. Front Psychol. 2014. PMID: 25071676 Free PMC article.
-
Sex, health and behaviour: Sexual reproduction might have emerged to provide better immunity against pathogens and further evolved to select for behaviour.EMBO Rep. 2016 Jan;17(1):18-21. doi: 10.15252/embr.201541661. Epub 2015 Dec 8. EMBO Rep. 2016. PMID: 26647368 Free PMC article.
References
-
- Trowsdale J. 2011. The MHC, disease and selection. Immunol. Lett. 137, 1–810.1016/j.imlet.2011.01.002 (doi:10.1016/j.imlet.2011.01.002) - DOI - DOI - PubMed
-
- Eizaguirre C, Lenz TL, Kalbe M, Milinski M. 2012. Rapid and adaptive evolution of MHC genes under parasite selection in experimental vertebrate populations. Nat. Commun. 3, 621.10.1038/ncomms1632 (doi:10.1038/ncomms1632) - DOI - DOI - PMC - PubMed
-
- Kubinak JL, Ruff JS, Hyzer CW, Slev PR, Potts WK. 2012. Experimental viral evolution to specific host MHC genotypes reveals fitness and virulence trade-offs in alternative MHC types. Proc. Natl Acad. Sci. USA 109, 3422–342710.1073/pnas.1112633109 (doi:10.1073/pnas.1112633109) - DOI - DOI - PMC - PubMed
-
- Nikolich-Žugich J, Fremont DH, Miley MJ, Messaoudi I. 2004. The role of MHC polymorphism in anti-microbial resistance. Microbes Infect. 6, 501–51210.1016/j.micinf.2004.01.006 (doi:10.1016/j.micinf.2004.01.006) - DOI - DOI - PubMed
-
- Piertney SB, Oliver MK. 2006. The evolutionary ecology of the major histocompatibility complex. Heredity 96, 7–2110.1038/sj.hdy.6800724 (doi:10.1038/sj.hdy.6800724) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

