Enzyme-linked immunosorbent assay using recombinant SAG1 antigen to detect Toxoplasma gondii-specific immunoglobulin G antibodies in human sera and saliva
- PMID: 23345586
- PMCID: PMC3623404
- DOI: 10.1128/CVI.00512-12
Enzyme-linked immunosorbent assay using recombinant SAG1 antigen to detect Toxoplasma gondii-specific immunoglobulin G antibodies in human sera and saliva
Abstract
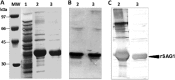
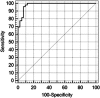
Serologic detection of Toxoplasma gondii IgG antibodies is widely accepted as a means to determine immune status and susceptibility to Toxoplasma infection during pregnancy. However, current commercial kits present some drawbacks, such as a requirement for whole-parasite antigen preparation or interassay variability. To address these problems, the purpose of this study was to produce a whole sequence of the recombinant T. gondii SAG1 antigen (rSAG1) to assess its diagnostic performance in Toxoplasma IgG screening and to explore a saliva-based method as a noninvasive alternative to serum-based testing. rSAG1 was expressed in recombinant bacteria as inclusion bodies, purified through one-step affinity chromatography, and refolded in native form by dialysis. A large amount was obtained, and the specific antigen immunoreactivity was confirmed by immunoblotting. Two rSAG1-based enzyme-linked immunosorbent assays (ELISAs) applied to paired serum and saliva samples were designed. The rSAG1-based ELISA evaluation consisted of testing intrinsic sensitivity and specificity of 49 serum samples from patients immune to toxoplasmosis and 42 serum samples from nonimmune controls identified by routinely used kits. To assess agreement between serum-based and saliva-based tests, the positive percent agreement (PPA) and negative percent agreement (NPA) between the 2 tests were estimated. The rSAG1 serum-based ELISA detected specific IgG with 100% sensitivity and specificity. The PPA and NPA between the serum-based and saliva-based tests varied according to the selected optical density threshold in saliva. Thus, for a selected cutoff of 0.14, the PPA was 100% and the NPA was 88.1%, whereas for a selected cutoff of 0.29, the PPA was 67.3% and the NPA was 100%.
Figures

Similar articles
-
Evaluation of recombinant SAG1, SAG2, and SAG3 antigens for serodiagnosis of toxoplasmosis.Korean J Parasitol. 2014 Apr;52(2):137-42. doi: 10.3347/kjp.2014.52.2.137. Epub 2014 Apr 18. Korean J Parasitol. 2014. PMID: 24850956 Free PMC article.
-
Toxoplasma gondii: Recombinant GRA5 antigen for detection of immunoglobulin G antibodies using enzyme-linked immunosorbent assay.Exp Parasitol. 2010 Mar;124(3):272-8. doi: 10.1016/j.exppara.2009.10.010. Epub 2009 Oct 27. Exp Parasitol. 2010. PMID: 19874823
-
Application of Toxoplasma gondii-specific SAG1, GRA7 and BAG1 proteins in serodiagnosis of animal toxoplasmosis.Front Cell Infect Microbiol. 2022 Dec 15;12:1029768. doi: 10.3389/fcimb.2022.1029768. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36590582 Free PMC article.
-
Recombinant proteins in the diagnosis of toxoplasmosis.APMIS. 2010 Aug;118(8):529-42. doi: 10.1111/j.1600-0463.2010.02629.x. APMIS. 2010. PMID: 20666734 Review.
-
Toxoplasma gondii recombinant antigens as tools for serodiagnosis of human toxoplasmosis: current status of studies.Clin Vaccine Immunol. 2013 Sep;20(9):1343-51. doi: 10.1128/CVI.00117-13. Epub 2013 Jun 19. Clin Vaccine Immunol. 2013. PMID: 23784855 Free PMC article. Review.
Cited by
-
Epidemiological survey of Toxoplasma gondii and Neospora caninum infections in dairy goats in Central-Southern Taiwan.J Vet Med Sci. 2020 Oct 30;82(10):1537-1544. doi: 10.1292/jvms.20-0116. Epub 2020 Sep 4. J Vet Med Sci. 2020. PMID: 32893199 Free PMC article.
-
Development of a Competitive Cystatin C-Specific Bioassay Suitable for Repetitive Measurements.PLoS One. 2016 Jan 22;11(1):e0147177. doi: 10.1371/journal.pone.0147177. eCollection 2016. PLoS One. 2016. PMID: 26799562 Free PMC article.
-
Key Limitations and New Insights Into the Toxoplasma gondii Parasite Stage Switching for Future Vaccine Development in Human, Livestock, and Cats.Front Cell Infect Microbiol. 2020 Nov 25;10:607198. doi: 10.3389/fcimb.2020.607198. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33324583 Free PMC article. Review.
-
Use of IgG in oral fluid to monitor infants with suspected congenital toxoplasmosis.Clin Vaccine Immunol. 2015 Apr;22(4):398-403. doi: 10.1128/CVI.00552-14. Epub 2015 Feb 4. Clin Vaccine Immunol. 2015. PMID: 25651923 Free PMC article.
-
Salivary IgA against sporozoite-specific embryogenesis-related protein (TgERP) in the study of horizontally transmitted toxoplasmosis via T. gondii oocysts in endemic settings.Epidemiol Infect. 2016 Sep;144(12):2568-77. doi: 10.1017/S0950268816000960. Epub 2016 May 12. Epidemiol Infect. 2016. PMID: 27169485 Free PMC article.
References
-
- Montoya JG, Remington JS. 2008. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 47:554–566 - PubMed
-
- Wong SY, Remington JS. 1994. Toxoplasmosis in pregnancy. Clin. Infect. Dis. 18:853–861 - PubMed
-
- Bouratbine A, Siala E, Chahed MK, Aoun K, Ben Ismail R. 2001. Sero-epidemiologic profile of toxoplasmosis in northern Tunisia. Parasite 8:61–66 - PubMed
-
- Kravetz JD, Federman DG. 2005. Toxoplasmosis in pregnancy. Am. J. Med. 118:212–216 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

