Identification of VAR2CSA domain-specific inhibitory antibodies of the Plasmodium falciparum erythrocyte membrane protein 1 using a novel flow cytometry assay
- PMID: 23345587
- PMCID: PMC3592344
- DOI: 10.1128/CVI.00638-12
Identification of VAR2CSA domain-specific inhibitory antibodies of the Plasmodium falciparum erythrocyte membrane protein 1 using a novel flow cytometry assay
Abstract
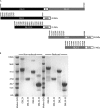
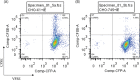
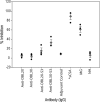
VAR2CSA, a member of the Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) family, is a leading candidate for use in vaccines to protect first-time mothers from placental malaria (PM). VAR2CSA, which is comprised of a series of six Duffy binding-like (DBL) domains, binds chondroitin sulfate A (CSA) on placental syncytiotrophoblast. Several recombinant DBL domains have been shown to bind CSA. In order to identify and develop recombinant proteins suitable for clinical development, DBL2X and DBL3X, as well as their respective third subdomain (S3) from the FCR3 parasite clone, were expressed in Escherichia coli, refolded, and purified. All but DBL3X-S3 recombinant proteins bound to CSA expressed on Chinese hamster ovary (CHO)-K1 cells but not to CHO-pgsA745 cells, which are CSA negative as determined by flow cytometry. All but DBL3X-S3 bound to CSA on chondroitin sulfate proteoglycan (CSPG) as determined by surface plasmon resonance (SPR) analysis. Purified IgG from rats and rabbits immunized with these four recombinant proteins bound homologous and some heterologous parasite-infected erythrocytes (IE). Using a novel flow cytometry inhibition-of-binding assay (flow-IBA), antibodies against DBL3X-S3 inhibited 35% and 45% of IE binding to CSA on CHO-K1 cells compared to results for soluble CSA (sCSA) and purified multigravida (MG) IgG, respectively, from areas in Tanzania to which malaria is endemic. Antibodies generated against the other domains provided little or no inhibition of IE binding to CSA on CHO-K1 cells as determined by the flow cytometry inhibition-of-binding assay. These results demonstrate for the first time the ability to identify antibodies to VAR2CSA DBL domains and subdomains capable of inhibiting VAR2CSA parasite-IE binding to CSA by flow cytometry. The flow cytometry inhibition-of-binding assay was robust and provided an accurate, reproducible, and reliable means to identify blocking of IE binding to CSA and promises to be significant in the development of a vaccine to protect pregnant women.
Figures



References
-
- Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82: 77– 87 - PubMed
-
- Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, Peterson DS. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82: 89– 100 - PubMed
-
- Costa FT, Avril M, Nogueira PA, Gysin J. 2006. Cytoadhesion of Plasmodium falciparum-infected erythrocytes and the infected placenta: a two-way pathway. Braz. J. Med. Biol. Res. 39: 1525– 1536 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

