Pancreatic digestive enzyme blockade in the intestine increases survival after experimental shock
- PMID: 23345609
- PMCID: PMC4643000
- DOI: 10.1126/scitranslmed.3005046
Pancreatic digestive enzyme blockade in the intestine increases survival after experimental shock
Abstract
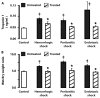
Shock, sepsis, and multiorgan failure are associated with inflammation, morbidity, and high mortality. The underlying pathophysiological mechanism is unknown, but evidence suggests that pancreatic enzymes in the intestinal lumen autodigest the intestine and generate systemic inflammation. Blocking these enzymes in the intestine reduces inflammation and multiorgan dysfunction. We investigated whether enzymatic blockade also reduces mortality after shock. Three rat shock models were used here: hemorrhagic shock, peritonitis shock induced by placement of cecal material into the peritoneum, and endotoxin shock. One hour after initiation of hemorrhagic, peritonitis, or endotoxin shock, animals were administered one of three different pancreatic enzyme inhibitors--6-amidino-2-naphtyl p-guanidinobenzoate dimethanesulfate, tranexamic acid, or aprotinin--into the lumen of the small intestine. In all forms of shock, blockade of digestive proteases with protease inhibitor attenuated entry of digestive enzymes into the wall of the intestine and subsequent autodigestion and morphological damage to the intestine, lung, and heart. Animals treated with protease inhibitors also survived in larger numbers than untreated controls over a period of 12 weeks. Surviving animals recovered completely and returned to normal weight within 14 days after shock. The results suggest that the active and concentrated digestive enzymes in the lumen of the intestine play a central role in shock and multiorgan failure, which can be treated with protease inhibitors that are currently available for use in the clinic.
Conflict of interest statement
Figures



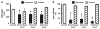
Similar articles
-
Pancreatic digestive enzyme blockade in the small intestine prevents insulin resistance in hemorrhagic shock.Shock. 2014 Jan;41(1):55-61. doi: 10.1097/SHK.0000000000000048. Shock. 2014. PMID: 24088998 Free PMC article.
-
Pancreatic enzymes generate cytotoxic mediators in the intestine.Shock. 2007 Mar;27(3):296-304. doi: 10.1097/01.shk.0000235139.20775.7f. Shock. 2007. PMID: 17304111
-
Generation of in vivo activating factors in the ischemic intestine by pancreatic enzymes.Proc Natl Acad Sci U S A. 2000 Feb 15;97(4):1772-7. doi: 10.1073/pnas.97.4.1772. Proc Natl Acad Sci U S A. 2000. PMID: 10677533 Free PMC article.
-
Autodigestion: Proteolytic Degradation and Multiple Organ Failure in Shock.Shock. 2016 May;45(5):483-9. doi: 10.1097/SHK.0000000000000544. Shock. 2016. PMID: 26717111 Free PMC article. Review.
-
A journey with Tony Hugli up the inflammatory cascade towards the auto-digestion hypothesis.Int Immunopharmacol. 2007 Dec 20;7(14):1845-51. doi: 10.1016/j.intimp.2007.07.015. Epub 2007 Aug 9. Int Immunopharmacol. 2007. PMID: 18039521 Free PMC article. Review.
Cited by
-
Volatile Decay Products in Breath During Peritonitis Shock are Attenuated by Enteral Blockade of Pancreatic Digestive Proteases.Shock. 2017 Nov;48(5):571-575. doi: 10.1097/SHK.0000000000000888. Shock. 2017. PMID: 28498300 Free PMC article.
-
Cathepsin K cleavage of angiopoietin-2 creates detrimental Tie2 antagonist fragments in sepsis.J Clin Invest. 2025 Mar 3;135(8):e174135. doi: 10.1172/JCI174135. eCollection 2025 Apr 15. J Clin Invest. 2025. PMID: 40029709 Free PMC article.
-
Therapeutic Potential of Mesenchymal Stromal Cell-Derived Extracellular Vesicles in the Prevention of Organ Injuries Induced by Traumatic Hemorrhagic Shock.Front Immunol. 2021 Sep 29;12:749659. doi: 10.3389/fimmu.2021.749659. eCollection 2021. Front Immunol. 2021. PMID: 34659252 Free PMC article. Review.
-
Digestive Enzyme Activity and Protein Degradation in Plasma of Heart Failure Patients.Cell Mol Bioeng. 2021 Aug 13;14(6):583-596. doi: 10.1007/s12195-021-00693-w. eCollection 2021 Dec. Cell Mol Bioeng. 2021. PMID: 34900012 Free PMC article.
-
The autodigestion hypothesis for shock and multi-organ failure.Ann Biomed Eng. 2014 Feb;42(2):405-14. doi: 10.1007/s10439-013-0891-6. Epub 2013 Aug 30. Ann Biomed Eng. 2014. PMID: 23989761 Free PMC article. Review.
References
-
- Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: A common mediator. Annu Rev Biochem. 1988;57:505–518. - PubMed
-
- Waxman K. Shock: Ischemia, reperfusion, and inflammation. New Horiz. 1996;4:153–160. - PubMed
-
- Liu SF, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. - PubMed
-
- Barroso-Aranda J, Schmid-Schönbein GW. Transformation of neutrophils as indicator of irreversibility in hemorrhagic shock. Am J Physiol. 1989;257:H846–H852. - PubMed
-
- Rice TW, Bernard GR. Therapeutic intervention and targets for sepsis. Annu Rev Med. 2005;56:225–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

