A non-catalytic role of DNA polymerase η in recruiting Rad18 and promoting PCNA monoubiquitination at stalled replication forks
- PMID: 23345618
- PMCID: PMC3597682
- DOI: 10.1093/nar/gkt016
A non-catalytic role of DNA polymerase η in recruiting Rad18 and promoting PCNA monoubiquitination at stalled replication forks
Abstract
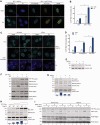
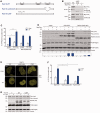
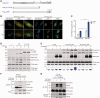
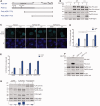
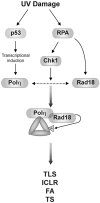
Trans-lesion DNA synthesis (TLS) is a DNA damage-tolerance mechanism that uses low-fidelity DNA polymerases to replicate damaged DNA. The inherited cancer-propensity syndrome xeroderma pigmentosum variant (XPV) results from error-prone TLS of UV-damaged DNA. TLS is initiated when the Rad6/Rad18 complex monoubiquitinates proliferating cell nuclear antigen (PCNA), but the basis for recruitment of Rad18 to PCNA is not completely understood. Here, we show that Rad18 is targeted to PCNA by DNA polymerase eta (Polη), the XPV gene product that is mutated in XPV patients. The C-terminal domain of Polη binds to both Rad18 and PCNA and promotes PCNA monoubiquitination, a function unique to Polη among Y-family TLS polymerases and dissociable from its catalytic activity. Importantly, XPV cells expressing full-length catalytically-inactive Polη exhibit increased recruitment of other error-prone TLS polymerases (Polκ and Polι) after UV irradiation. These results define a novel non-catalytic role for Polη in promoting PCNA monoubiquitination and provide a new potential mechanism for mutagenesis and genome instability in XPV individuals.
Figures

Similar articles
-
c-Jun N-terminal kinase-mediated Rad18 phosphorylation facilitates Polη recruitment to stalled replication forks.Mol Biol Cell. 2012 May;23(10):1943-54. doi: 10.1091/mbc.E11-10-0829. Epub 2012 Mar 28. Mol Biol Cell. 2012. PMID: 22456510 Free PMC article.
-
Structural basis for novel interactions between human translesion synthesis polymerases and proliferating cell nuclear antigen.J Biol Chem. 2009 Apr 17;284(16):10552-60. doi: 10.1074/jbc.M809745200. Epub 2009 Feb 10. J Biol Chem. 2009. PMID: 19208623 Free PMC article.
-
Rad6/Rad18 Competes with DNA Polymerases η and δ for PCNA Encircling DNA.Biochemistry. 2020 Feb 4;59(4):407-416. doi: 10.1021/acs.biochem.9b00938. Epub 2020 Jan 10. Biochemistry. 2020. PMID: 31887036
-
Diverse roles of RAD18 and Y-family DNA polymerases in tumorigenesis.Cell Cycle. 2018;17(7):833-843. doi: 10.1080/15384101.2018.1456296. Epub 2018 May 8. Cell Cycle. 2018. PMID: 29683380 Free PMC article. Review.
-
Regulation of DNA damage tolerance in mammalian cells by post-translational modifications of PCNA.Mutat Res. 2017 Oct;803-805:82-88. doi: 10.1016/j.mrfmmm.2017.06.004. Epub 2017 Jun 21. Mutat Res. 2017. PMID: 28666590 Review.
Cited by
-
High RAD18 Expression is Associated with Disease Progression and Poor Prognosis in Patients with Gastric Cancer.Ann Surg Oncol. 2020 Oct;27(11):4360-4368. doi: 10.1245/s10434-020-08518-2. Epub 2020 Apr 30. Ann Surg Oncol. 2020. PMID: 32356270
-
Detection of endogenous translesion DNA synthesis in single mammalian cells.Cell Rep Methods. 2023 Jun 14;3(6):100501. doi: 10.1016/j.crmeth.2023.100501. eCollection 2023 Jun 26. Cell Rep Methods. 2023. PMID: 37426760 Free PMC article.
-
Characterisation of the spectrum and genetic dependence of collateral mutations induced by translesion DNA synthesis.PLoS Genet. 2022 Feb 7;18(2):e1010051. doi: 10.1371/journal.pgen.1010051. eCollection 2022 Feb. PLoS Genet. 2022. PMID: 35130276 Free PMC article.
-
Microsatellite break-induced replication generates highly mutagenized extrachromosomal circular DNAs.NAR Cancer. 2024 Jun 8;6(2):zcae027. doi: 10.1093/narcan/zcae027. eCollection 2024 Jun. NAR Cancer. 2024. PMID: 38854437 Free PMC article.
-
A neomorphic cancer cell-specific role of MAGE-A4 in trans-lesion synthesis.Nat Commun. 2016 Jul 5;7:12105. doi: 10.1038/ncomms12105. Nat Commun. 2016. PMID: 27377895 Free PMC article.
References
-
- Friedberg EC. DNA damage and repair. Nature. 2003;421:436–440. - PubMed
-
- Friedberg EC, Wagner R, Radman M. Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science. 2002;296:1627–1630. - PubMed
-
- Friedberg EC, Fischhaber PL, Kisker C. Error-prone DNA polymerases: novel structures and the benefits of infidelity. Cell. 2001;107:9–12. - PubMed
-
- Goodman MF. Error-prone repair DNA polymerases in prokaryotes and eukaryotes. Annu. Rev. Biochem. 2002;71:17–50. - PubMed
-
- Ohmori H, Friedberg EC, Fuchs RP, Goodman MF, Hanaoka F, Hinkle D, Kunkel TA, Lawrence CW, Livneh Z, Nohmi T, et al. The Y-family of DNA polymerases. Mol. Cell. 2001;8:7–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

