Intestinal injury can be reduced by intra-arterial postischemic perfusion with hypertonic saline
- PMID: 23345943
- PMCID: PMC3547561
- DOI: 10.3748/wjg.v19.i2.209
Intestinal injury can be reduced by intra-arterial postischemic perfusion with hypertonic saline
Abstract
Aim: To investigate the effect of local intestinal perfusion with hypertonic saline (HTS) on intestinal ischemia-reperfusion injury (IRI) in both ex vivo and in vivo rat models.
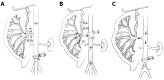
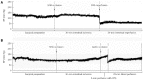
Methods: All experiments were performed on male Wistar rats anesthetized with pentobarbital sodium given intraperitoneally at a dose of 60 mg/kg. Ex vivo vascularly perfused rat intestine was subjected to 60-min ischemia and either 30-min reperfusion with isotonic buffer (controls), or 5 min with HTS of 365 or 415 mOsm/L osmolarity (HTS(365mOsm) or HTS(415mOsm), respectively) followed by 25-min reperfusion with isotonic buffer. The vascular intestinal perfusate flow (IPF) rate was determined by collection of the effluent from the portal vein in a calibrated tube. Spontaneous intestinal contraction rate was monitored throughout. Irreversible intestinal injury or area of necrosis (AN) was evaluated histochemically using 2.3.5-triphenyltetrazolium chloride staining. In vivo, 30-min ischemia was followed by either 30-min blood perfusion or 5-min reperfusion with HTS(365mOsm) through the superior mesenteric artery (SMA) followed by 25-min blood perfusion. Arterial blood pressure (BP) was measured in the common carotid artery using a miniature pressure transducer. Histological injury was evaluated in both preparations using the Chui score.
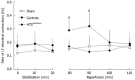
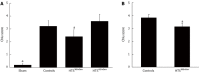
Results: Ex vivo, intestinal IRI resulted in a reduction in the IPF rate during reperfusion (P < 0.05 vs sham). The postischemic recovery of the IPF rate did not differ between the controls and the HTS(365mOsm) group. In the HTS(415mOsm) group, postischemic IPF rates were lower than in the controls and the HTS(365mOsm) group (P < 0.05). The intestinal contraction rate was similar at baseline in all groups. An increase in this parameter was observed during the first 10 min of reperfusion in the control group as compared to the sham-treated group, but no such increase was seen in the HTS(365mOsm) group. In controls, AN averaged 14.8% ± 5.07% of the total tissue volume. Administration of HTS(365mOsm) for 5 min after 60-min ischemia resulted in decrease in AN (5.1% ± 1.20% vs controls, P < 0.01). However, perfusion of the intestine with the HTS of greater osmolarity (HTS(415mOsm)) failed to protect the intestine from irreversible injury. The Chiu score was lower in the HTS(365mOsm) group in comparison with controls (2.4 ± 0.54 vs 3.2 ± 0.44, P = 0.042), while intestinal perfusion with HTS(415mOsm) failed to improve the Chiu score. Intestinal reperfusion with HTS(365mOsm) in the in vivo series secured rapid recovery of BP after its transient fall, whereas in the controls no recovery was seen. The Chiu score was lower in the HTS(365mOsm) group vs controls (3.1 ± 0.26 and 3.8 ± 0.22, P = 0.0079 respectively,), although the magnitude of the effect was lower than in the ex vivo series.
Conclusion: Brief intestinal postischemic perfusion with HTS(365mOsm) through the SMA followed by blood flow restoration is a protective procedure that could be used for the prevention of intestinal IRI.
Keywords: Acute mesenteric ischemia; Hypertonic saline; Intestinal ischemia-reperfusion injury; Intestinal perfusate flow rate; Perfusion; Superior mesenteric artery.
Figures


References
-
- Wyers MC. Acute mesenteric ischemia: diagnostic approach and surgical treatment. Semin Vasc Surg. 2010;23:9–20. - PubMed
-
- Berland T, Oldenburg WA. Acute mesenteric ischemia. Curr Gastroenterol Rep. 2008;10:341–346. - PubMed
-
- Arthurs ZM, Titus J, Bannazadeh M, Eagleton MJ, Srivastava S, Sarac TP, Clair DG. A comparison of endovascular revascularization with traditional therapy for the treatment of acute mesenteric ischemia. J Vasc Surg. 2011;53:698–704; discussion 704-705. - PubMed
-
- Vollmar B, Menger MD. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbecks Arch Surg. 2011;396:13–29. - PubMed
-
- Takeshita M, Tani T, Harada S, Hayashi H, Itoh H, Tajima H, Ohnishi I, Takamura H, Fushida S, Kayahara M. Role of transcription factors in small intestinal ischemia-reperfusion injury and tolerance induced by ischemic preconditioning. Transplant Proc. 2010;42:3406–3413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

