Partially hydrolyzed guar gum in pediatric functional abdominal pain
- PMID: 23345946
- PMCID: PMC3547554
- DOI: 10.3748/wjg.v19.i2.235
Partially hydrolyzed guar gum in pediatric functional abdominal pain
Abstract
Aim: To assess the effects of partially hydrolyzed guar gum (PHGG) diet supplement in pediatric chronic abdominal pain (CAP) and irritable bowel syndrome (IBS).
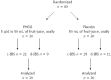
Methods: A randomized, double-blind pilot study was performed in sixty children (8-16 years) with functional bowel disorders, such as CAP or IBS, diagnosed according to Rome III criteria. All patients underwent ultrasound, blood and stool examinations to rule out any organic disease. Patients were allocated to receive PHGG at dosage of 5 g/d (n = 30) or placebo (fruit-juice n = 30) for 4 wk. The evaluation of the efficacy of fiber supplement included IBS symptom severity score (Birmingham IBS Questionnaire), severity of abdominal pain (Wong-Baker Face Pain Rating Score) and bowel habit (Bristol Stool Scale). Symptom scores were completed at 2, 4, and 8 wk. The change from baseline in the symptom severity scale at the end of treatment and at 4 wk follow-up after treatment was the primary endpoint. The secondary endpoint was to evaluate compliance to supplementation with the PHGG in the pediatric population. Differences within groups during the treatment period and follow-up were evaluated by the Wilcoxon signed-rank test.
Results: The results of the study were assessed considering some variables, such as frequency and intensity of symptoms with modifications of the bowel habit. Both groups were balanced for baseline characteristics and all patients completed the study. Group A (PHGG group) presented a higher level of efficacy compared to group B (control group), (43% vs 5%, P = 0.025) in reducing clinical symptoms with modification of Birmingham IBS score (median 0 ± 1 vs 4 ± 1, P = 0.025), in intensity of CAP assessed with the Wong-Baker Face Pain Rating Score and in normalization of bowel habit evaluated with the Bristol Stool Scale (40% vs 13.3%, P = 0.025). In IBS subgroups, statistical analysis shown a tendency toward normalization of bowel movements, but there was no difference in the prevalence of improvement in two bowel habit subsets. PHGG was therefore better tolerated without any adverse effects.
Conclusion: Although the cause of pediatric functional gastrointestinal disorders is not known, the results show that complementary therapy with PHGG may have beneficial effects on symptom control.
Keywords: Fiber diet; Functional bowel disorders; Partially hydrolyzed guar gum; Pediatric chronic abdominal pain.
Figures
References
-
- Clouse RE, Mayer EA, Aziz Q, Drossman DA, Dumitrascu DL, Mönnikes H, Naliboff BD. Functional abdominal pain syndrome. Gastroenterology. 2006;130:1492–1497. - PubMed
-
- Starfield B, Hoekelman RA, McCormick M, Benson P, Mendenhall RC, Moynihan C, Radecki S. Who provides health care to children and adolescents in the United States? Pediatrics. 1984;74:991–997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous