Clinical outcomes and predictive factors in oral corticosteroid-refractory active ulcerative colitis
- PMID: 23345950
- PMCID: PMC3547555
- DOI: 10.3748/wjg.v19.i2.265
Clinical outcomes and predictive factors in oral corticosteroid-refractory active ulcerative colitis
Abstract
Aim: To evaluate the clinical outcomes and prognostic factors after intravenous corticosteroids following oral corticosteroid failure in active ulcerative colitis patients.
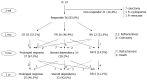
Methods: Consecutive patients with moderate to severe ulcerative colitis who had been treated with a course of intravenous corticosteroids after oral corticosteroid therapy failure between January 1996 and July 2010 were recruited at Severance Hospital, Seoul, South Korea. The disease activity was measured by the Mayo score, which consists of stool frequency, rectal bleeding, mucosal appearance at flexible sigmoidoscopy, and Physician Global Assessment. We retrospectively evaluated clinical outcomes at two weeks, one month, three months, and one year after the initiation of intravenous corticosteroid therapy. Two weeks outcomes were classified as responders or non-responders. One month, three month and one year outcomes were classified into prolonged response, steroid dependency, and refractoriness.
Results: Our study included a total of 67 eligible patients. At two weeks, 56 (83.6%) patients responded to intravenous corticosteroids. At one month, complete remission was documented in 18 (32.1%) patients and partial remission in 26 (46.4%). Eleven patients (19.7%) were refractory to the treatment. At three months and one year, we found 37 (67.3%) and 25 (46.3%) patients in prolonged response, ten (18.2%) and 23 (42.6%) patients in corticosteroid dependency, 8 (14.5%) and 6 (11.1%) patients with no response, respectively. Total 9 patients were underwent elective proctocolectomy within 1 year. The duration of oral corticosteroid therapy (> 14 d vs ≤ 14 d, P = 0.049) and lower hemoglobin level (≤ 11.0 mg/dL vs >11.0 mg/dL, P = 0.02) were found to be poor prognostic factors for response at two weeks. For one year outcome, univariate analysis revealed that only a partial Mayo score (≥ 6 vs <6, P = 0.057) was found to be associated with a poor response.
Conclusion: The duration of oral corticosteroid therapy and lower hemoglobin level were strongly associated with poor outcome.
Keywords: Clinical outcome; Corticosteroid; Prognosis; Ulcerative colitis.
Figures
References
-
- Abu-Suboh Abadía M, Casellas F, Vilaseca J, Malagelada JR. Response of first attack of inflammatory bowel disease requiring hospital admission to steroid therapy. Rev Esp Enferm Dig. 2004;96:539–44; 544-7. - PubMed
-
- Faubion WA, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. - PubMed
-
- Domènech E, Garcia-Planella E, Bernal I, Rosinach M, Cabré E, Fluvià L, Boix J, Gassull MA. Azathioprine without oral ciclosporin in the long-term maintenance of remission induced by intravenous ciclosporin in severe, steroid-refractory ulcerative colitis. Aliment Pharmacol Ther. 2002;16:2061–2065. - PubMed
-
- Kohn A, Daperno M, Armuzzi A, Cappello M, Biancone L, Orlando A, Viscido A, Annese V, Riegler G, Meucci G, et al. Infliximab in severe ulcerative colitis: short-term results of different infusion regimens and long-term follow-up. Aliment Pharmacol Ther. 2007;26:747–756. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical