New and emerging treatments for estrogen receptor-positive breast cancer: focus on everolimus
- PMID: 23345981
- PMCID: PMC3549674
- DOI: 10.2147/TCRM.S30349
New and emerging treatments for estrogen receptor-positive breast cancer: focus on everolimus
Abstract
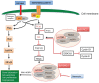
Management of patients with metastatic hormone receptor-positive breast cancer poses a challenge due to the inevitable development of endocrine resistance. Hormone resistance is associated with a complex interaction of the estrogen receptor with growth factors, transmembrane receptors, and intracellular growth cascades. The PI3K/Akt/mTOR pathway plays a major role in hormone resistance and proliferation of breast cancer. Preclinical and clinical data indicate that inhibitors of human epidermal growth factor receptor-2, epidermal growth factor receptor, insulin-like growth factor-1 receptor, and the mammalian target of rapamycin pathway may act synergistically with hormone therapy to circumvent endocrine resistance. Everolimus is currently approved for combination with exemestane in postmenopausal women with advanced hormone receptor-positive breast cancer. However, we still need to unfold the full potential of targeted agents in the hormone-refractory setting and to identify the subsets of patients who will benefit from combination hormonal therapy using targeted agents.
Keywords: estrogen receptor-positive breast cancer; everolimus; hormone resistance; inhibition; mammalian target of rapamycin.
Figures
References
-
- Siegel R, Naishadham D, Ahmedin Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- National Cancer Institute. SEER statistic fact sheet: breast cancer. [Accessed August 6, 2012]. Available from: http://seer.cancer.gov/statfacts/html/breast.html.
-
- Harvey JM, Clark GM, Osborne CK, Alfred DC. Estrogen receptor status by immunohistochemistry is superior to ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. - PubMed
-
- Colleoni M, Gelber S, Coates AS, et al. Influence of endocrine-related factors on response to perioperative chemotherapy for patients with node-negative breast cancer. J Clin Oncol. 2001;19:4141–4149. - PubMed
-
- Winer EP. Optimizing endocrine therapy for breast cancer. J Clin Oncol. 2005;23:1609–1610. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

