Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain
- PMID: 23346046
- PMCID: PMC3548228
- DOI: 10.3389/fncel.2012.00070
Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain
Abstract
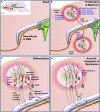
Neural stem/precursor cells in the adult brain reside in the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus. These cells primarily generate neuroblasts that normally migrate to the olfactory bulb (OB) and the dentate granule cell layer respectively. Following brain damage, such as traumatic brain injury, ischemic stroke or in degenerative disease models, neural precursor cells from the SVZ in particular, can migrate from their normal route along the rostral migratory stream (RMS) to the site of neural damage. This neural precursor cell response to neural damage is mediated by release of endogenous factors, including cytokines and chemokines produced by the inflammatory response at the injury site, and by the production of growth and neurotrophic factors. Endogenous hippocampal neurogenesis is frequently also directly or indirectly affected by neural damage. Administration of a variety of factors that regulate different aspects of neural stem/precursor biology often leads to improved functional motor and/or behavioral outcomes. Such factors can target neural stem/precursor proliferation, survival, migration and differentiation into appropriate neuronal or glial lineages. Newborn cells also need to subsequently survive and functionally integrate into extant neural circuitry, which may be the major bottleneck to the current therapeutic potential of neural stem/precursor cells. This review will cover the effects of a range of intrinsic and extrinsic factors that regulate neural stem/precursor cell functions. In particular it focuses on factors that may be harnessed to enhance the endogenous neural stem/precursor cell response to neural damage, highlighting those that have already shown evidence of preclinical effectiveness and discussing others that warrant further preclinical investigation.
Keywords: gliogenesis; ischemic stroke; neural precursor cell (NPC); neural stem cell (NSC); neural stem/precursor cell (NSPC); neurogenesis; subventricular zone (SVZ); traumatic brain injury.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

