Carbocyclic analogues of inosine-5'-monophosphate: synthesis and biological activity
- PMID: 23346382
- PMCID: PMC3549521
Carbocyclic analogues of inosine-5'-monophosphate: synthesis and biological activity
Abstract
9-(4'-Phosphonomethoxy-2'-cyclopenten-1'-yl)hypoxanthine and 9-(4'-phosphonomethoxy-2',3'-dihydroxycyclopenten-1'-yl)hypoxanthine were synthesized as isosteric carbocyclic analogues of inosine-5'-monophosphate. The synthesized compounds were shown to be capable of inhibiting the activity of human type II inosine-5'-monophosphate dehydrogenase (IMPDH II) (IC(50 )= 500 µM) and to have no significant effects on the growth ofMycobacterium tuberculosis.
Keywords: Carbocyclic nucleosides; competitive inhibition; human IMPDH II,Mycobacterium tuberculosis; inosine-5’-monophosphate.
Figures
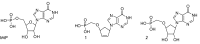

References
-
- Nair V., Shu Q.. Antiviral. Chem. Chemotherapy. 2007;18:245–258. - PubMed
-
- Shu Q., Nair V.. Med. Res. Rev. 2008;28(2) - PubMed
-
- Khandazhinskaya A.L., Shirokova E.A., Shipitsin A.V., Belanov E.F., Kukhanova M.K.. Collect. Chech. Chem. Commun. 2006;71:1107–1121.
-
- Andreevskaya S.N., Chernousova L.N., Smirnova T.G., Larionova E.E., Kuzmin A.V.. Problems of Tuberculosis and Lung Disease (Russian). 2006;12:43–48.
LinkOut - more resources
Full Text Sources
